Preparation method of Afatinib intermediate
A technology of compound and zinc powder, which is applied in the field of preparation of afatinib intermediates, can solve the problems of high production cost, low yield, and easy-to-corrosion reaction kettle, and achieve low production cost, low pollution, and high product yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
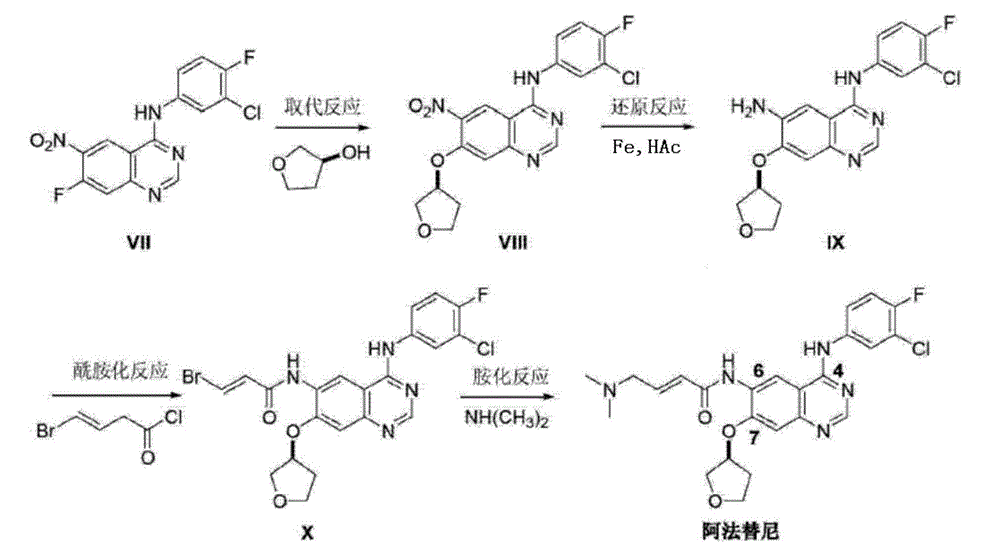
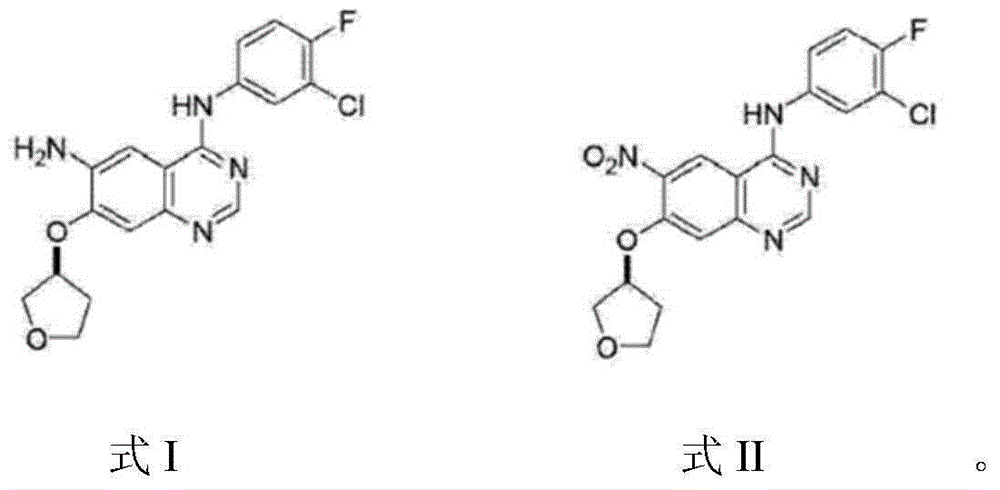
[0021] Take 1.0g (2.47mmol) 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, 20mL Add ethanol, 1.5mL glacial acetic acid, and 10mL water into the reaction flask, heat to reflux, add 0.50g (7.69mmol) of zinc powder in batches, keep stirring at reflux for 1h, filter while hot, rinse the filter cake with a small amount of ethanol, and wash the filtrate Distill under reduced pressure until the volume is reduced by about half, then cool naturally to room temperature, add ammonia water dropwise to neutral, filter with suction, rinse the filter cake with 20mL water to obtain off-white solid N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine. After drying and testing, mass: 0.79g, yield: 85.36%, purity 96.09%.
Embodiment 2
[0023] Take 1.0g (2.47mmol) 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline into 20mL Methanol, take a catalytic amount of calcium chloride dissolved in 10ml of water and add to the reaction flask, stir evenly, add 0.50g (7.69mmol) of zinc powder, keep stirring at reflux for 2h, filter while hot, rinse the filter cake with a small amount of methanol, and combine After the filtrate was poured into 50ml of water, a small amount of 10% NaOH was added dropwise until the solution was weakly alkaline, and solids were precipitated, filtered with suction, and the filter cake was rinsed with 10mL of water to obtain off-white solid N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine. After drying and testing, mass: 0.69g, yield: 74.53%, purity 97.32%.
Embodiment 3
[0025] Take 2.0g (4.94mmol) 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, 35mL Add ethanol, 6.5mL of concentrated hydrochloric acid, and 35mL of water into the reaction flask, heat to reflux, and slowly add 1.60g (24.7mmol) of zinc powder in batches. Keep stirring under reflux for 1 hour, filter while it is hot, rinse the filter cake with a small amount of ethanol, distill the filtrate under reduced pressure until the volume is reduced by half, cool naturally to room temperature, add ammonia water dropwise until neutral, filter with suction, and rinse the filter cake with 50mL water washed to give an off-white solid N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine. After drying and testing, mass: 1.55g, yield: 83.72%, purity 97.59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com