Method of Adjuvant Cancer Treatment
a cancer treatment and adjuvant technology, applied in the field of adjuvant cancer treatment, can solve the problems of high risk of local and distant relapse after definitive surgery, high risk of relapse, and limited therapeutic options in the adjuvant setting, and achieve the effect of increasing relapse-free survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
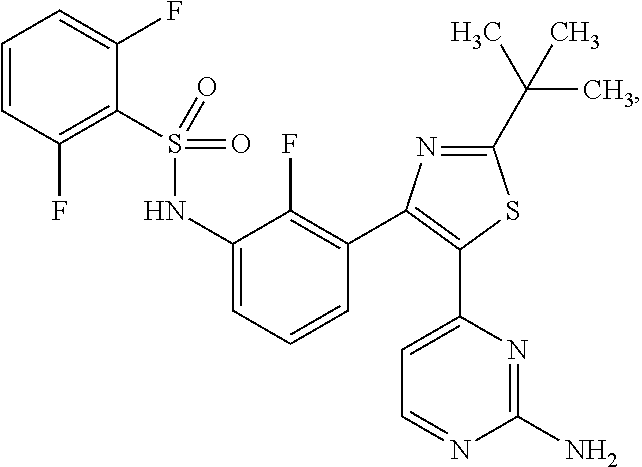
[0010]The RAS / RAF / MEK / ERK pathway (i.e., the MAP kinase pathway) is a critical proliferation pathway in many human cancers, including melanoma. Oncogenic mutations in BRAF signal through MEK1 and MEK2, and occurrence of this is an early event. This study will evaluate the combination of two small-molecules, oral agents, dabrafenib and trametinib. Dabrafenib is a potent and selective RAF kinase inhibitor of human wild type BRAF and CRAF enzymes as well as the mutant forms BRAFV600E, BRAFV600K and BRAFV600D. The mode of action of dabrafenib is consistent with competitive inhibition of adenosine triphosphate (ATP) binding. By contrast, trametinib is a reversible, highly selective, allosteric inhibitor of MEK1 and MEK2. Trametinib is non-competitive towards ATP and inhibits both MEK activation and kinase activity. Because BRAF and MEK are in the same pathway, and because MEK is a substrate of activated BRAF and other kinases that can be activated in presence of BRAF inhibition, inhibiti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com