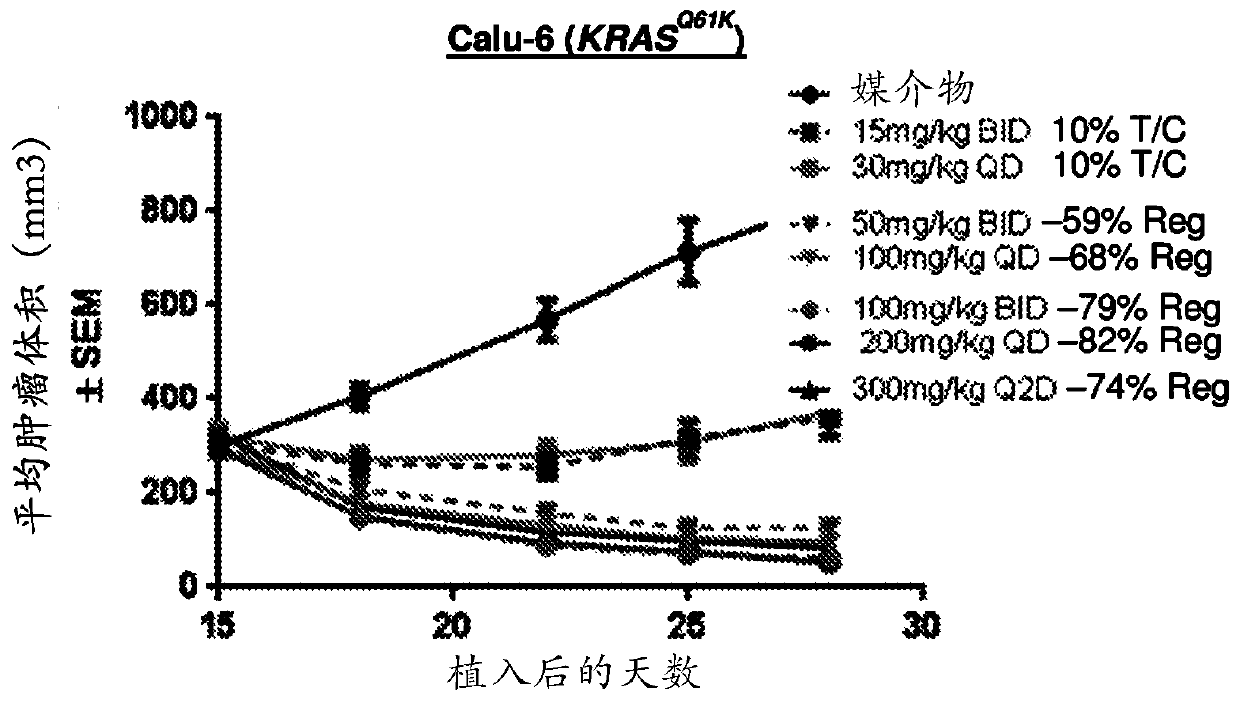
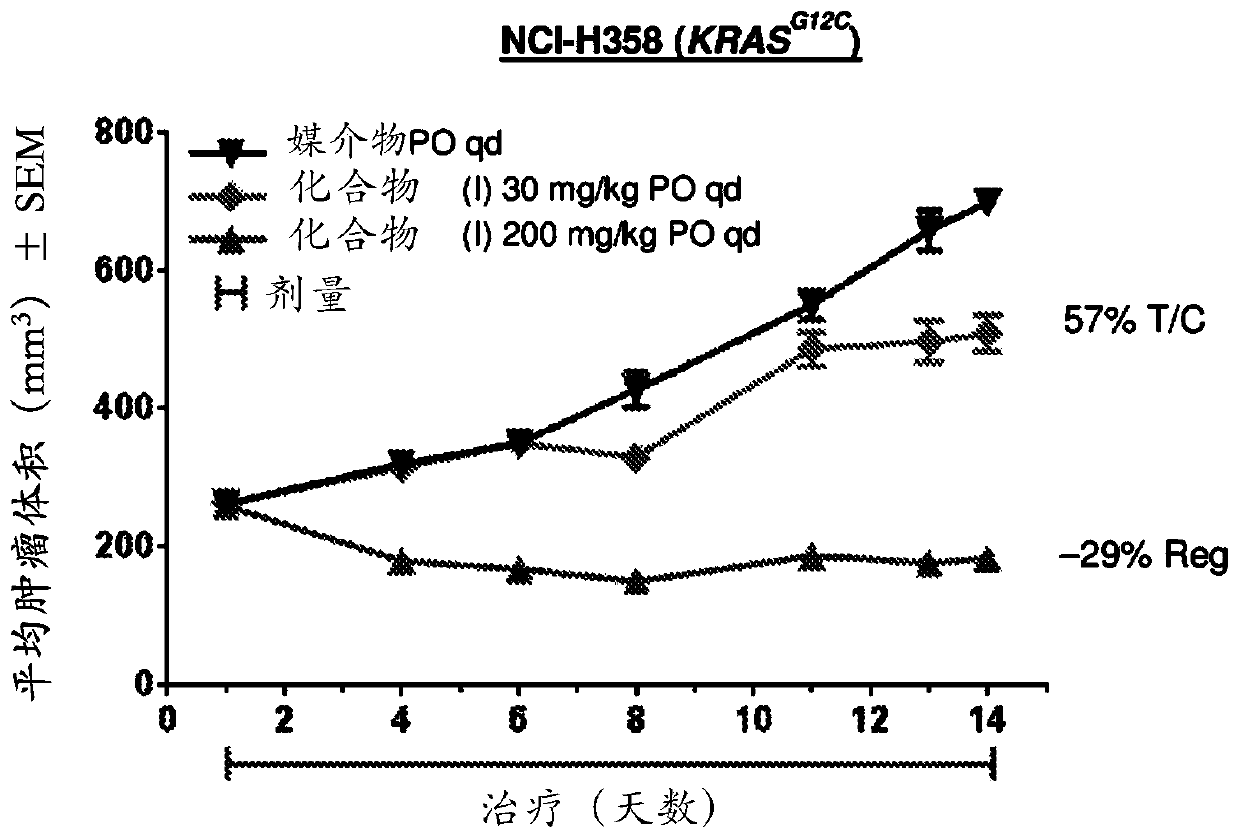
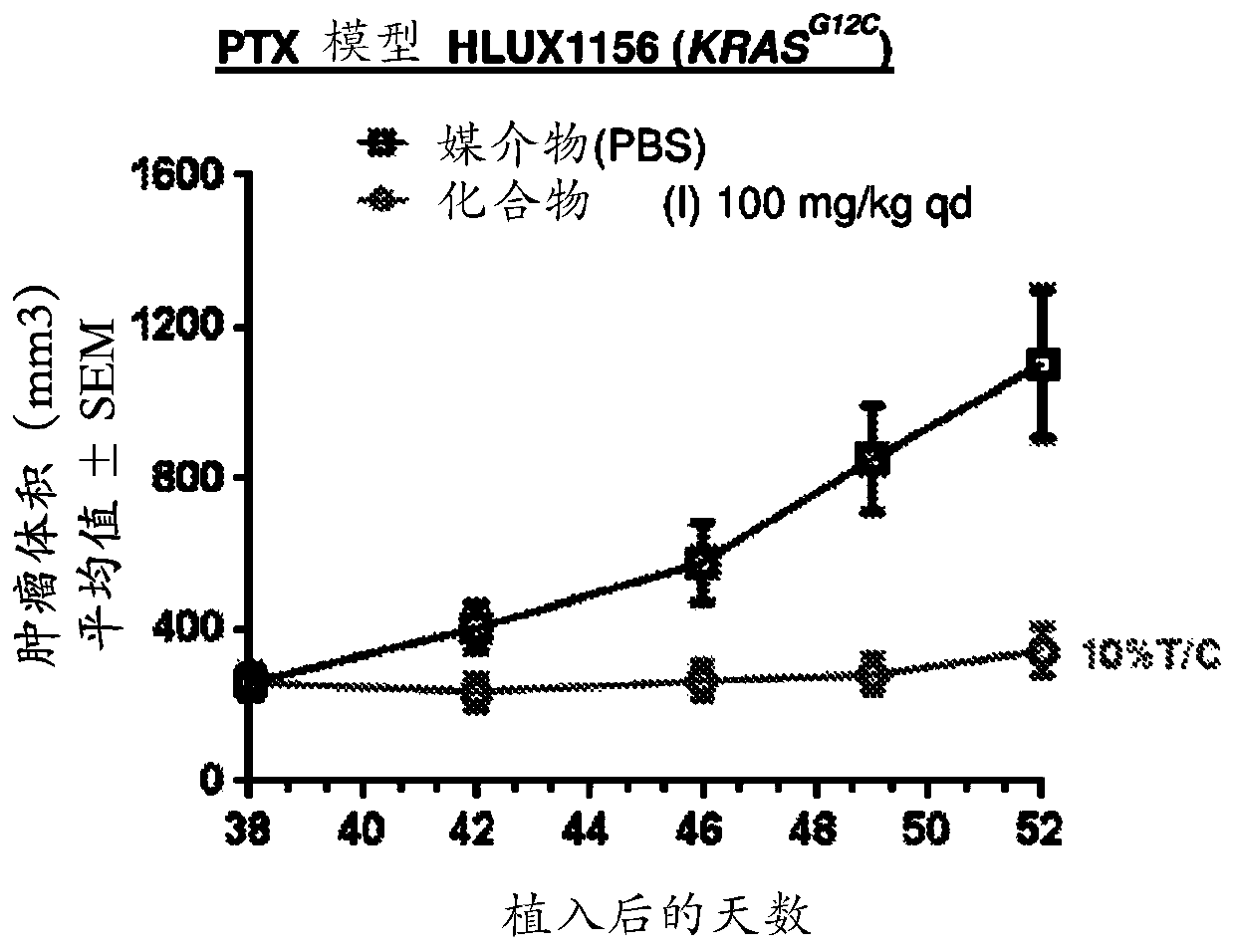
Combination therapy
A composition and compound technology, applied in the direction of drug combination, active ingredient of heterocyclic compound, active ingredient of amide, etc., can solve the problem of no targeted therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0204] Example 1 :N-(3-(2-(2-hydroxyethoxy)-6-morpholinopyridin-4-yl)-4-methylphenyl)-2-(trifluoromethyl)isonicotinamide
[0205] The compound with formula (I) is a morpholine substituted biaryl compound with the following structure
[0206]
example 1
[0209] Compounds of formula (I) are type II inhibitors of both b-Raf and c-Raf.
[0210] Table 1. The half-maximum inhibitory concentration (IC-50) of compounds of formula (I) on b-Raf and c-Raf
[0211] Compound b-RafIC-50(μM) c-Raf FLIC-50(μM) Compounds of formula (I)0.000730.00020
example 1
[0213] Compounds of formula (I) show activity in many human cancer cell lines expressing mutations in the MAPK pathway, as shown in the table below. For cell lines with at least one mutation in BRAF or RAS, the activity is particularly strong.
[0214] Table 2. The effect of compounds of formula (I) on the proliferation of a group of human cancer cell lines.
[0215]
[0216]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com