Application of trametinib in preparation of anti-arenavirus antiviral preparation
An arenavirus and antiviral technology, which is applied in the directions of antiviral agents, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of not obtaining approval for specific inhibition of arenaviruses, etc., and achieve a good therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0038] Experimental example 1 inhibits the infection effect of multiple arenavirus pseudoviruses
[0039] Operation method:
[0040] 1) In the experiment, Vero E6 cells and A549 cells were cultured in DMEM medium containing 10% fetal bovine serum, and the cells were digested the day before the experiment, and the cells were inoculated to 96 cells at a cell density of 250,000 cells / mL. in the orifice plate.
[0041] 2) Trametinib was diluted with 2% DMEM medium on the day of the experiment, and the concentrations of LASV, LUJV and VSV were: 75 μM, 15 μM, 3 μM, 0.6 μM, 0.12 μM, 0.024 μM. The concentrations used for LCMV, JUNV, GTOV, MACV, SBAV and CHAPV are: 100 μM, 50 μM, 25 μM, 12.5 μM, 6.25 μM, 3.125 μM, 1.5625 μM, 0.78125 μM, 0.390625 μM, and then transfer 30 μl trametinib To the cell wells, the drug was incubated with the cells for 1 h.
[0042] 3) Dilute the virus after 1 hour. Place 20 µL of the virus dilution into the cell wells. Virus infection 1h. The virus is a ...
experiment example 2
[0049] Experimental example 2 inhibits the infection effect of LUJV-GPC recombinant virus
[0050] Operation method:
[0051] 1) In the experiment, Vero E6 cells and A549 cells were cultured in DMEM medium containing 10% fetal bovine serum, and the cells were digested the day before the experiment, and the cells were seeded into 96-well plates at a cell density of 250,000 cells / ml .
[0052] 2) On the day of the experiment, trametinib was diluted in DMEM medium with 2% fetal bovine serum to use concentrations: 75 μM, 15 μM, 3 μM, 0.6 μM, 0.12 μM, 0.024 μM. Afterwards, 30 μL of the drug was transferred to the cell well, and the drug was incubated with the cells for 1 h.
[0053] 3) After 1 hour, the virus was diluted at an MOI=0.1. Place 20 µL of the virus dilution into the cell wells. Virus infection 1h.
[0054] 4) The 96-well plate was replaced with 2% fetal bovine serum DMEM containing corresponding concentration of drugs for culture.
[0055] 5) After 24 hours, the d...
experiment example 3
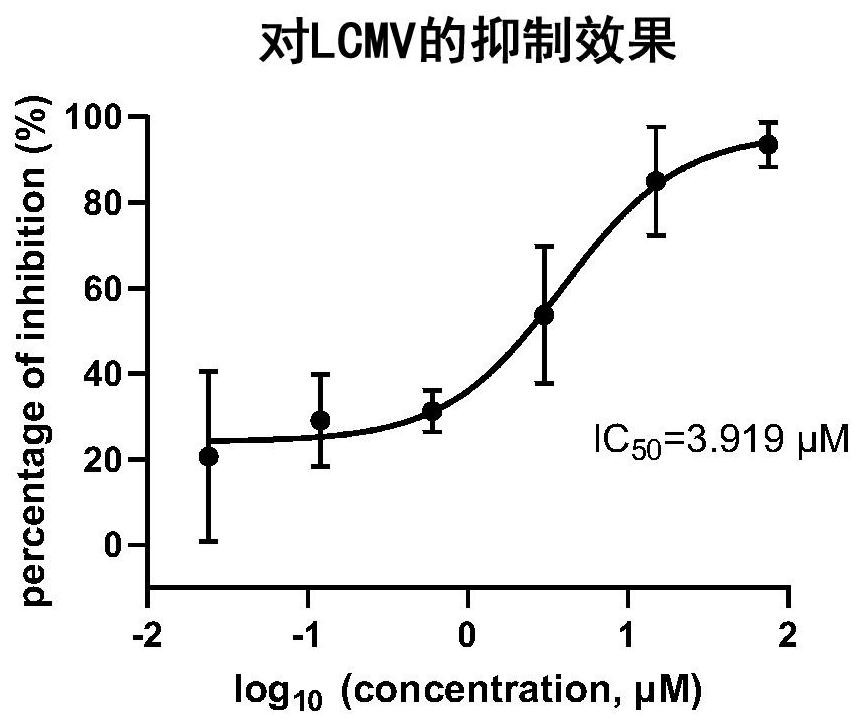
[0057] Experimental Example 3: Inhibition of LCMV virus infection effect
[0058] Operation method:
[0059] 1) In the experiment, Vero E6 cells were cultured in DMEM medium containing 10% fetal bovine serum, and the cells were digested and inoculated one day before the experiment.
[0060] 2) Dilute the cells at a density of 250000 cells / mL, and inoculate 500 μL per well into a 24-well plate.
[0061] 3) On the day of the experiment, 200 μL of trametinib at different dilution concentrations was used to incubate the cells for 1 h. The diluted concentrations of trametinib were 75 μM, 15 μM, 3 μM, 0.6 μM, 0.12 μM, and 0.024 μM, respectively.
[0062] 4) After incubation for 1 hour, the cells were infected at MOI=0.1, and the original medium was aspirated after the cells were infected for 1 hour, and replaced with the medium containing the drug.
[0063] 5) Cells were lysed with TRIzol lysate 24 hours after infection, and samples were collected. After the samples were collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com