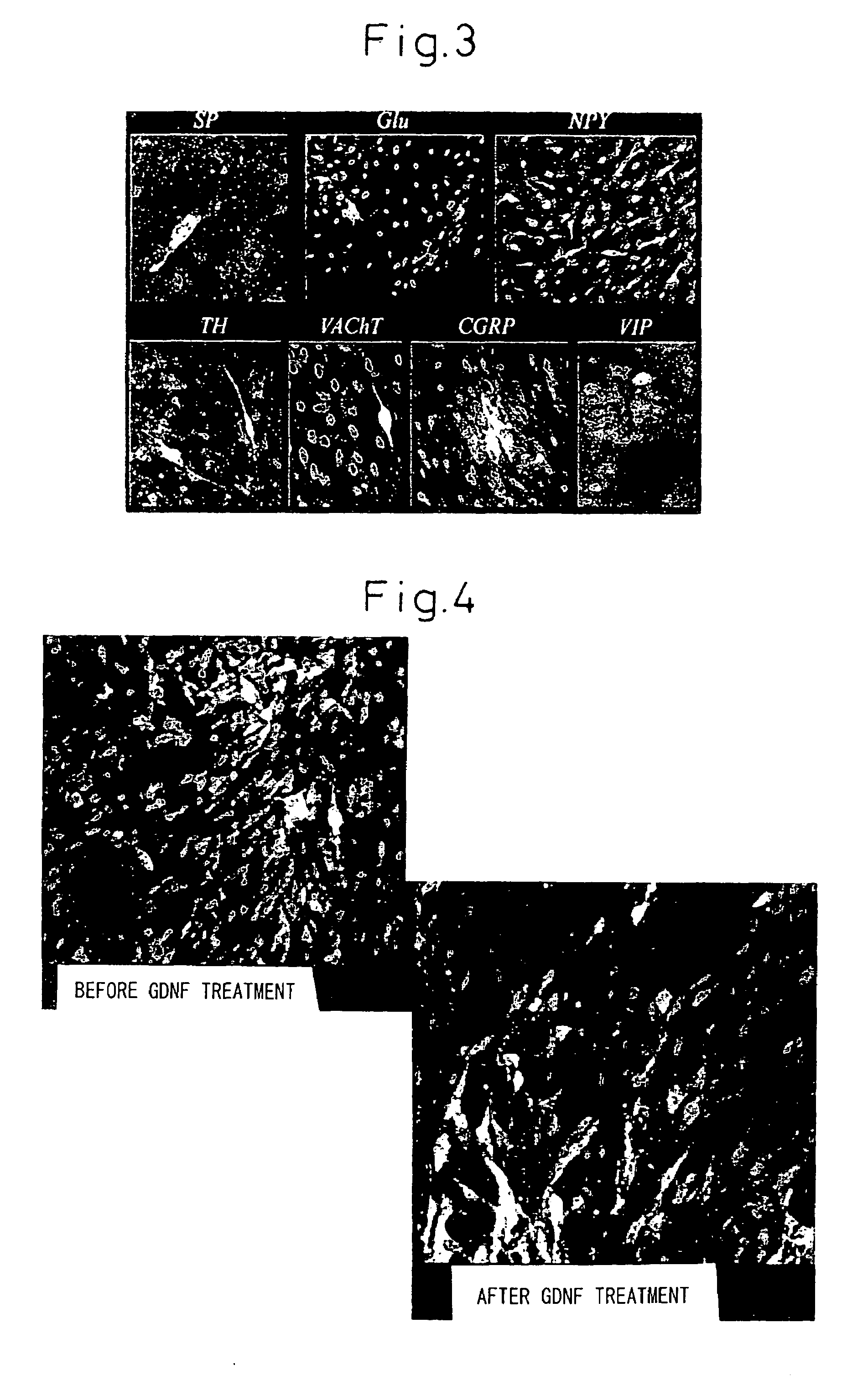
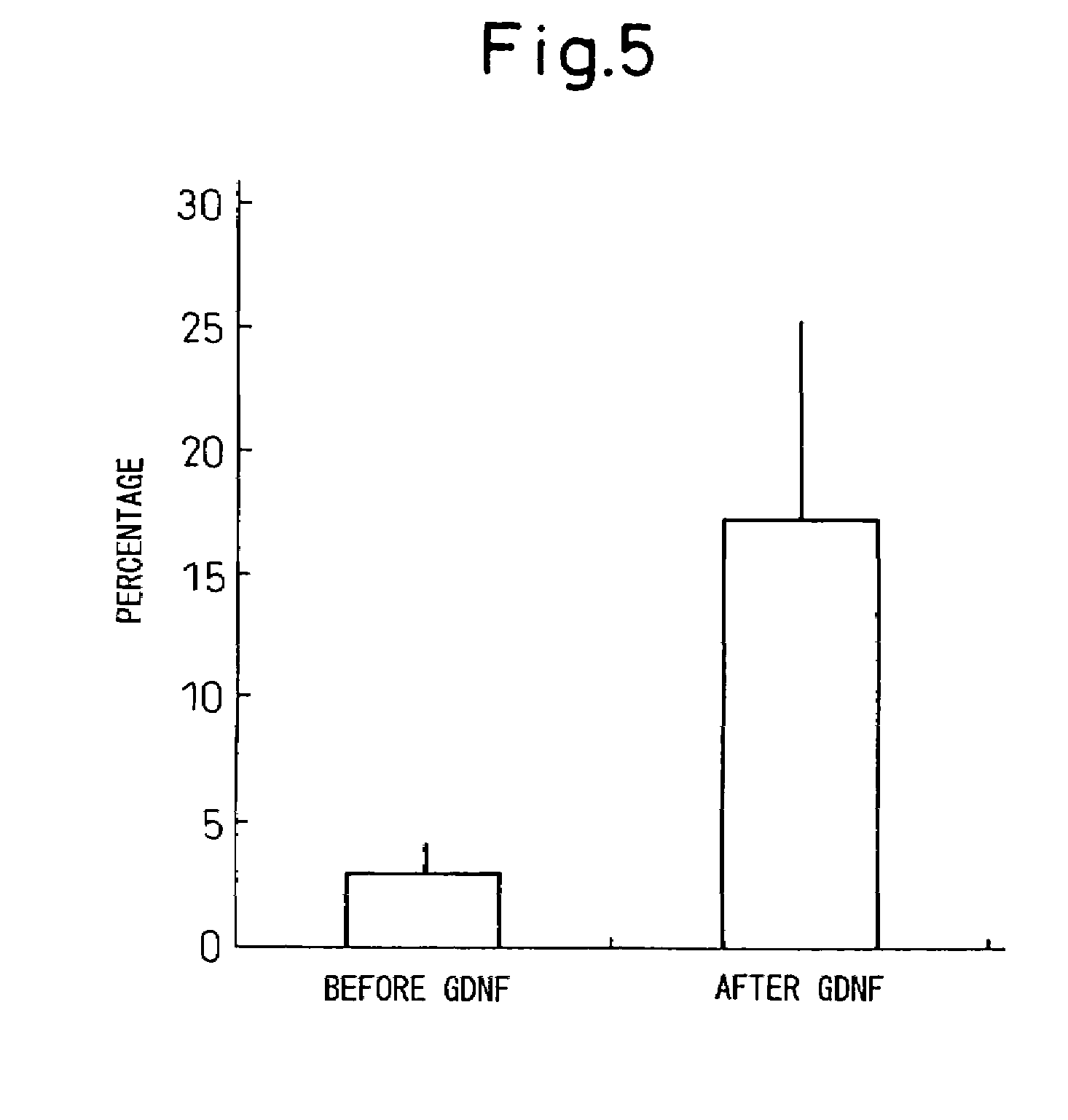
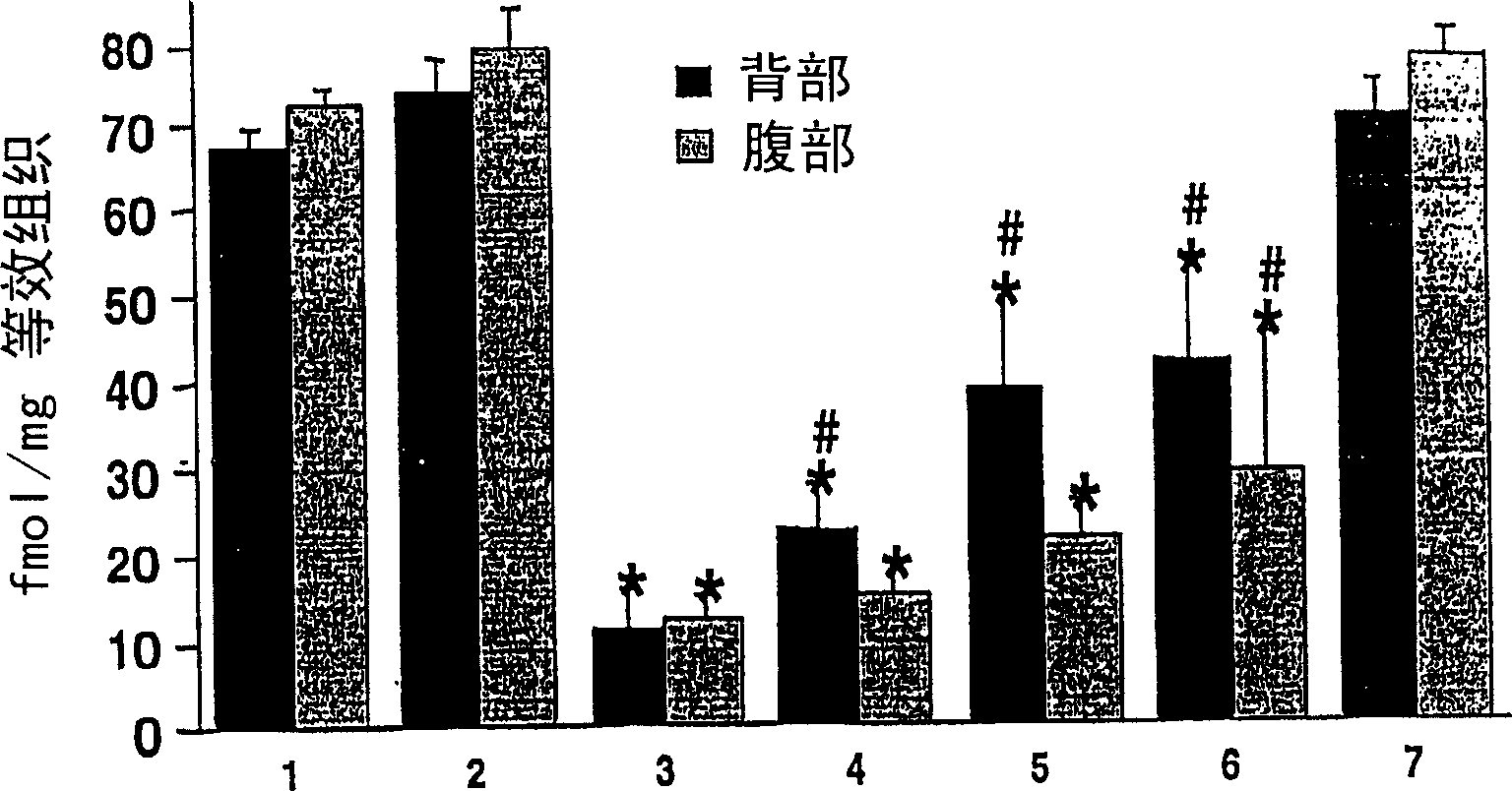
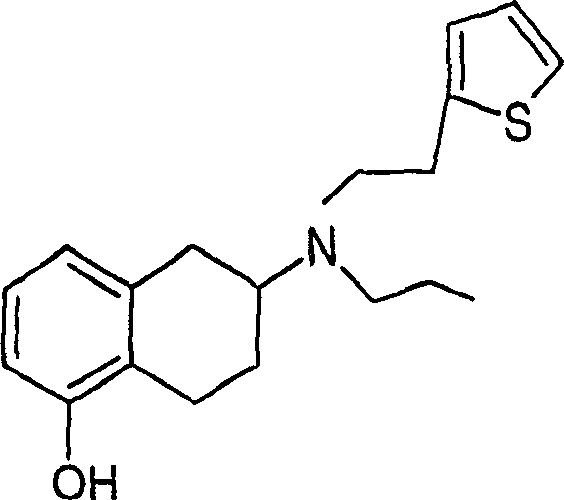
Patents
Literature
315 results about "Dopaminergic neuron" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods to improve neural outcome
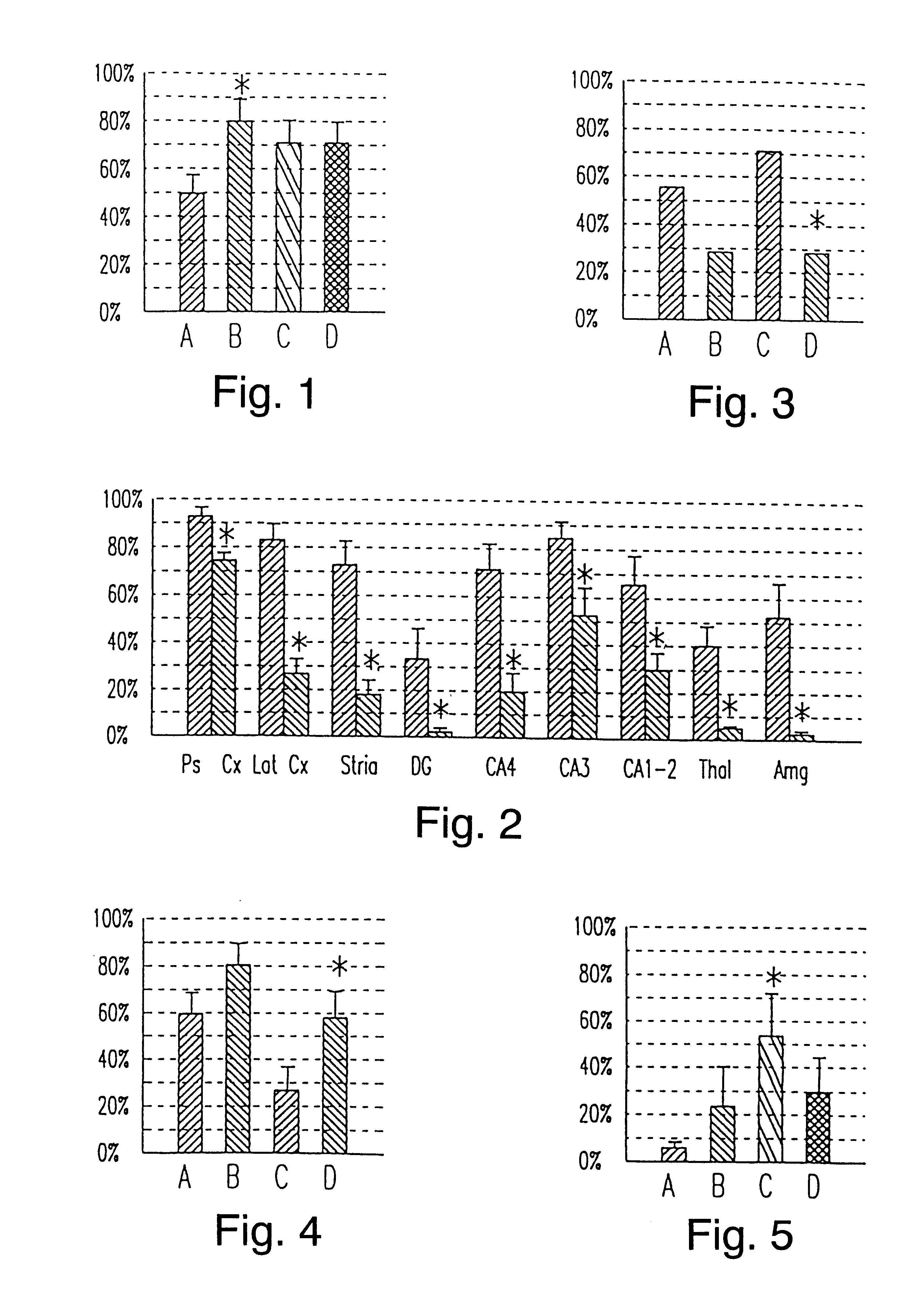
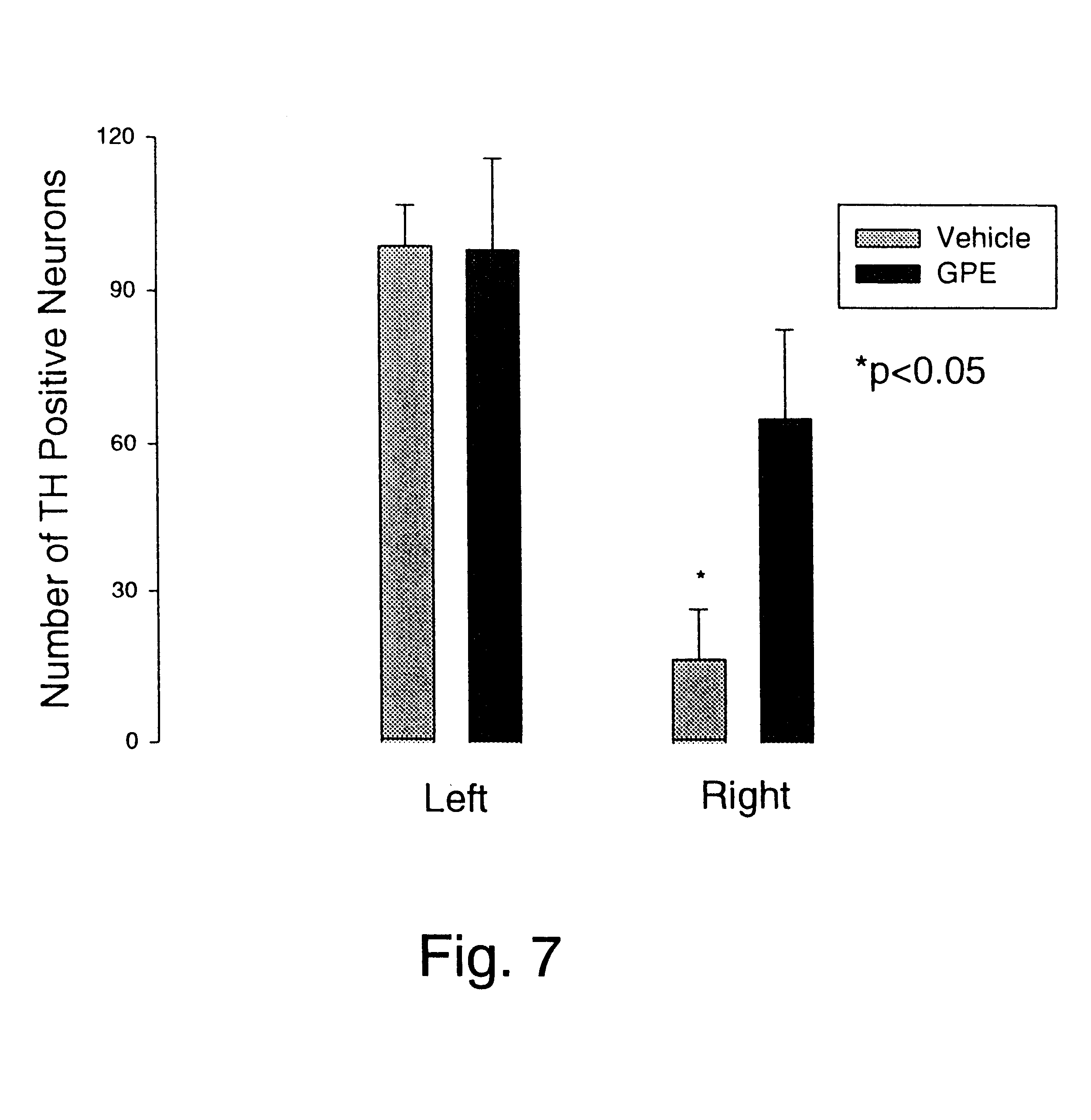
Disclosed herein is a method for protecting dopaminergic neurons of a mammal against death resulting from Parkinson's disease. The method comprises administering a neuroprotective amount of the tripeptide Gly-Pro-Glu.
Owner:NEUREN PHARMA LTD
Differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells by introduction of notch gene
There is provided a method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells by introduction of a Notch gene. Specifically, the invention provides a method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells in vitro, which method comprises introducing a Notch gene and / or a Notch signaling related gene into the cells, wherein the finally obtained differentiated cells are the result of cell division of the bone marrow stromal cells into which the Notch gene and / or Notch signaling related gene have been introduced. The invention also provides a method of inducing further differentiation of the differentiation-induced neural cells to dopaminergic neurons or acetylcholinergic neurons. The invention yet further provides a treatment method for neurodegenerative and skeletal muscle degenerative diseases which employs neural precursor cells, neural cells or skeletal muscle cells produced by the method of the invention.
Owner:SANBIO
Use of rotigotine for treatment or prevention of dopaminergic neurone loss
The invention relates to the use of rotigotine or salts thereof and prodrugs for the production of a medicament for the treatment or prevention of dopaminergic cell destruction in diseases which are connected to increased dopaminergic cell destruction. The invention also relates to the use of rotigotine as a medicament for the preventive treatment of Parkinson's disease.
Owner:UCB SA
Method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells by introduction of notch gene
There is provided a method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells by introduction of a Notch gene. Specifically, the invention provides a method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells in vitro, which method comprises introducing a Notch gene and / or a Notch signaling related gene into the cells, wherein the finally obtained differentiated cells are the result of cell division of the bone marrow stromal cells into which the Notch gene and / or Notch signaling related gene have been introduced. The invention also provides a method of inducing further differentiation of the differentiation-induced neural cells to dopaminergic neurons or acetylcholinergic neurons. The invention yet further provides a treatment method for neurodegenerative and skeletal muscle degenerative diseases which employs neural precursor cells, neural cells or skeletal muscle cells produced by the method of the invention.
Owner:SANBIO
Gene specifically expressed in postmitotic dopaminergic neuron precursor cells
InactiveUS20070122882A1Efficient separationEasy to prepareFungiBacteriaNeuro-degenerative diseaseNovel gene
A novel gene 65B13 expressed specifically and transiently in dopaminergic neuron precursor cells immediately after cell cycle exit was obtained by the present invention. The cellular expression of 65B13 can be used as an index to select cells that are suitable in terms of their safety, survival rate, and network formation ability, for transplant therapy of neurodegenerative diseases such as Parkinson's disease.
Owner:EISIA R&D MANAGEMENT CO LTD
Derivation of terminally differentiated dopaminergic neurons from human embryonic stem cells
InactiveUS20060211109A1Simple methodIncrease percentageNervous disorderCulture processNervous systemNeural cell
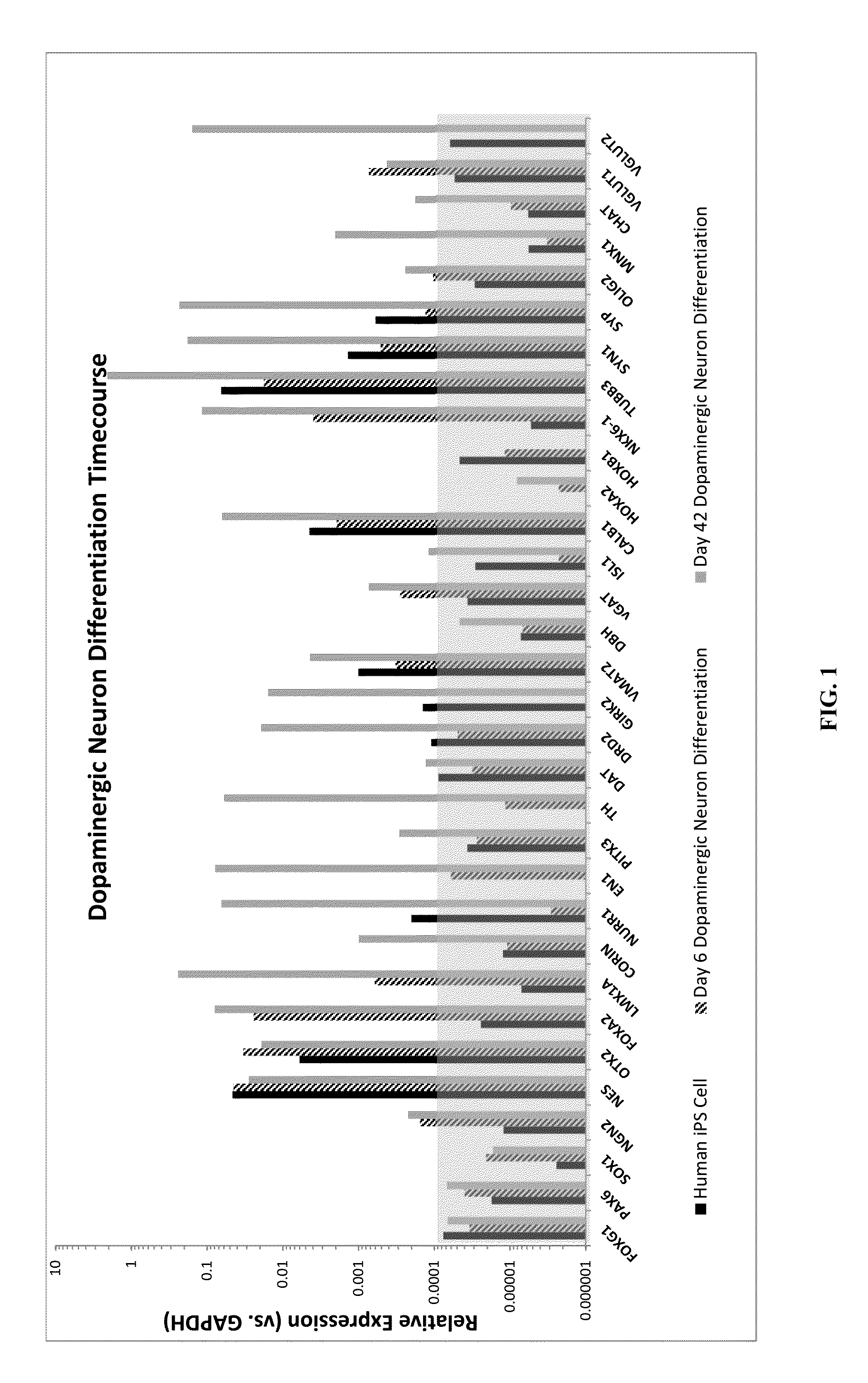
The present disclosure is directed to improved methods for efficiently producing neuroprogenitor cells and differentiated neural cells such as dopaminergic neurons and serotonergic neurons from pluripotent stem cells, for example human embryonic stem cells. Using the disclosed methods, cell populations containing a high proportion of cells positive for tyrosine hydroxylase, a specific marker for dopaminergic neurons, have been isolated. The neuroprogenitor cells and terminally differentiated cells of the present disclosure can be generated in large quantities, and therefore may serve as an excellent source for cell replacement therapy in neurological disorders such as Parkinson's disease.
Owner:RELIANCE LIFE SCI PVT
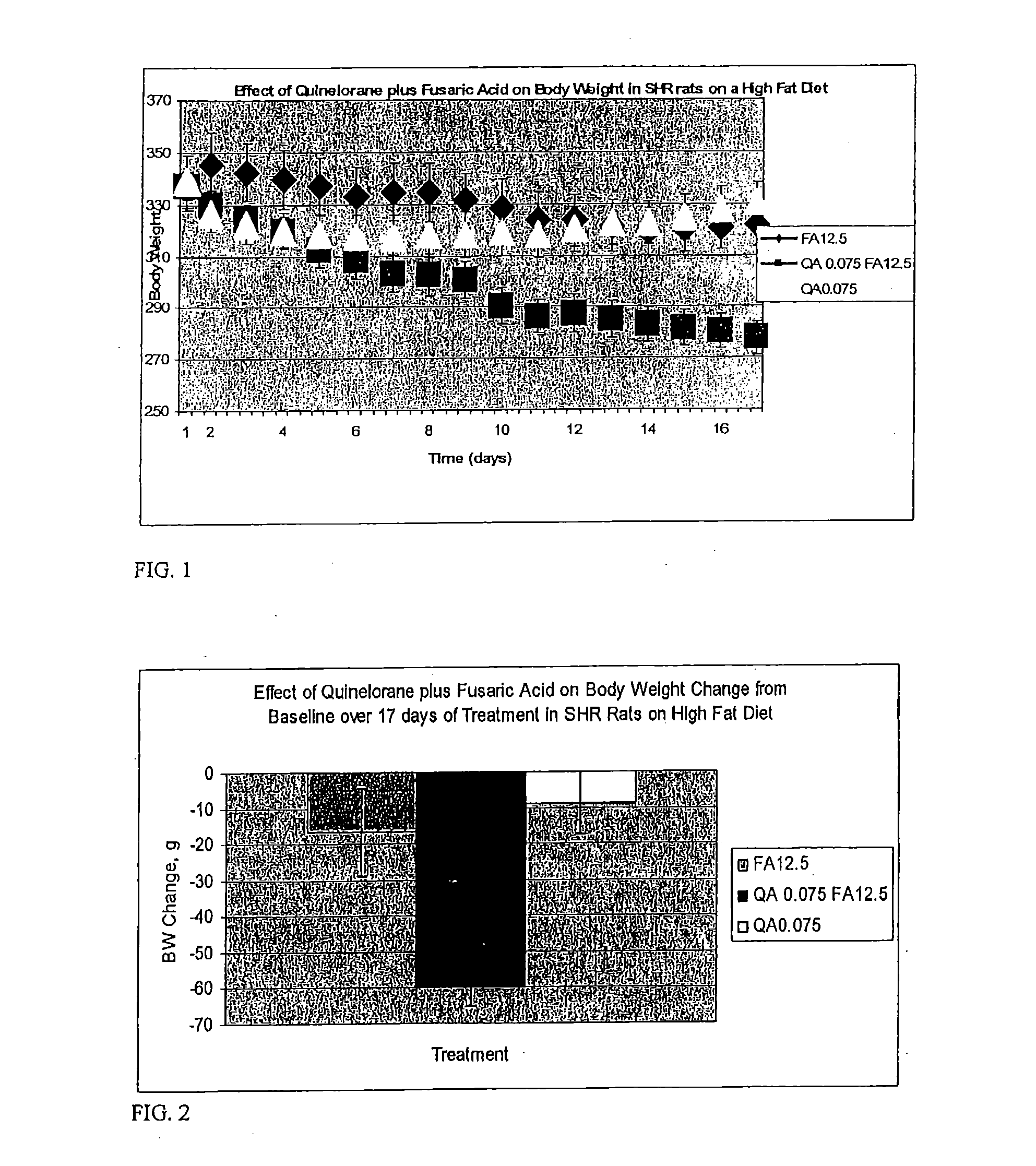
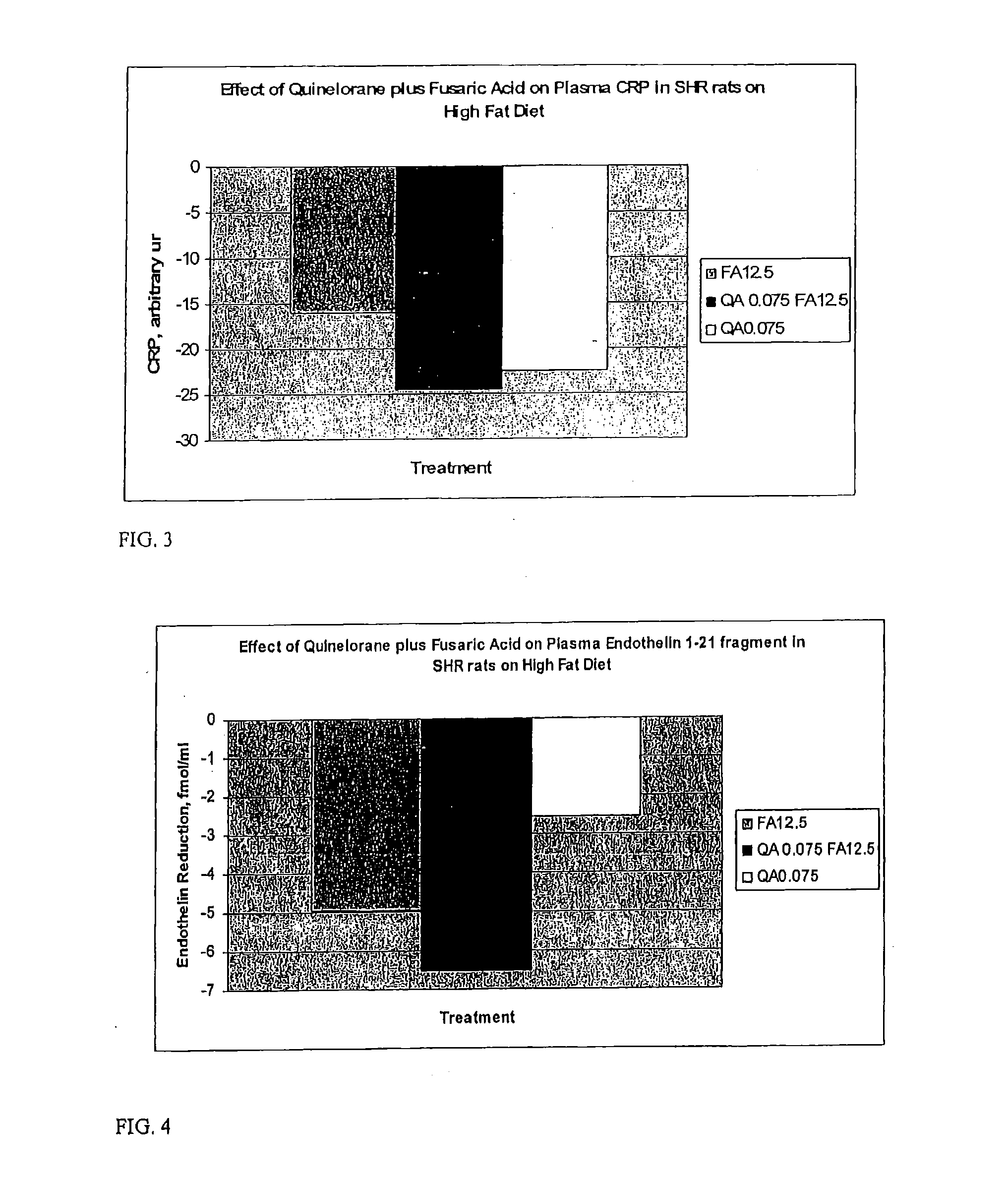
Therapeutic treatment for metabolic syndrome, type 2 diabetes, obesity, or prediabetes
ActiveUS20080293735A1Increases central neuronal dopamine activityReduced activityBiocidePeptide/protein ingredientsNoradrenergic neuronsPrediabetes
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic neuronal activity within the central nervous system and particularly the hypothalamus of the central nervous system of the patient.In another aspect, the present invention is directed to a method for treating a patient suffering from a metabolic disorder such as the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, and the metabolic sequale of these diseases including cardiovascular, cerebrovascular, renal and hepatic diseases, comprising the step of: administering to a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes a pharmaceutical composition comprising (1) at least one compound that stimulates an increase in central dopaminergic neuronal activity level in the subject, and (2) at least one compound that stimulates a decrease in central noradrenergic neuronal activity level in the subject. The present invention is also directed to pharmaceutical compositions that include the above compounds and a pharmaceutically acceptable carrier.
Owner:VEROSCI
Production of midbrain dopaminergic neurons and methods for the use thereof
Methods are provided for efficient production of midbrain dopaminergic (DA) neurons. In some aspects, methods involve differentiation and selection of DA neurons for a transgenic pluripotent cell population (e.g., cells comprising a selectable marker gene). Cell populations produced by the instant methods and methods of their use are likewise provided.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Therapeutic treatment for the metabolic syndrome and type 2 diabetes
The present invention is directed to a method for treating the metabolic syndrome or Type 2 diabetes, comprising the step of administering to a subject in need of such treatment a pharmaceutical composition comprising (1) at least one compound that stimulates an increase in central dopaminergic neuronal activity level in the subject, and (2) at least one compound that stimulates a decrease in central noradrenergic neuronal activity level in the subject. The present invention is also directed to a method for treating the metabolic syndrome or Type 2 diabetes, comprising the step of administering to a subject in need of such treatment at least one compound that simultaneously stimulates (1) an increase in central dopaminergic neuronal activity level, and (2) a decrease in central noradrenergic neuronal activity level. The present invention is also directed to pharmaceutical compositions that include the above compounds and a pharmaceutically acceptable carrier.
Owner:CINCOTTA ANTHONY H
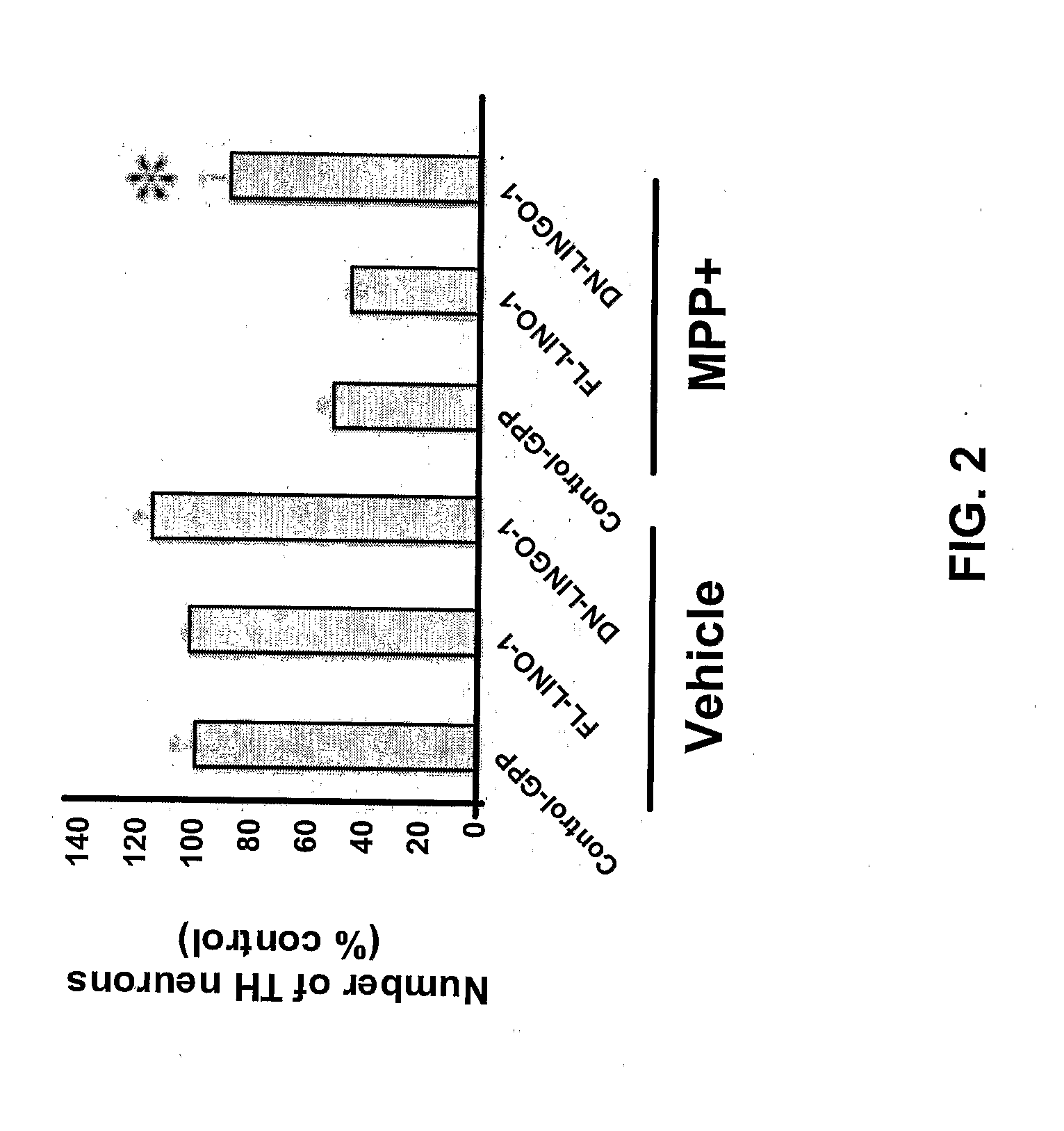
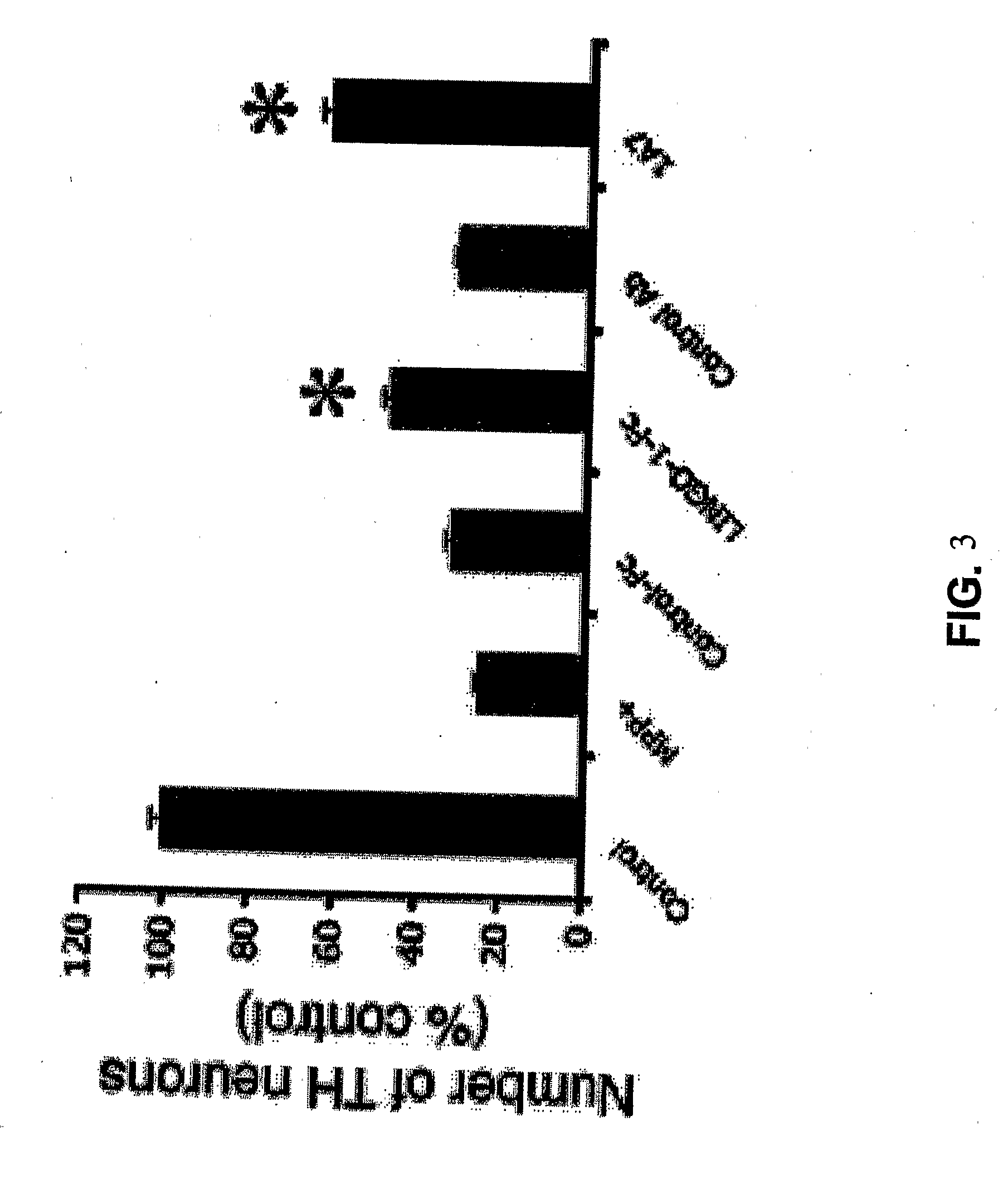
Methods for Promoting Neurite Outgrowth and Survival of Dopaminergic Neurons
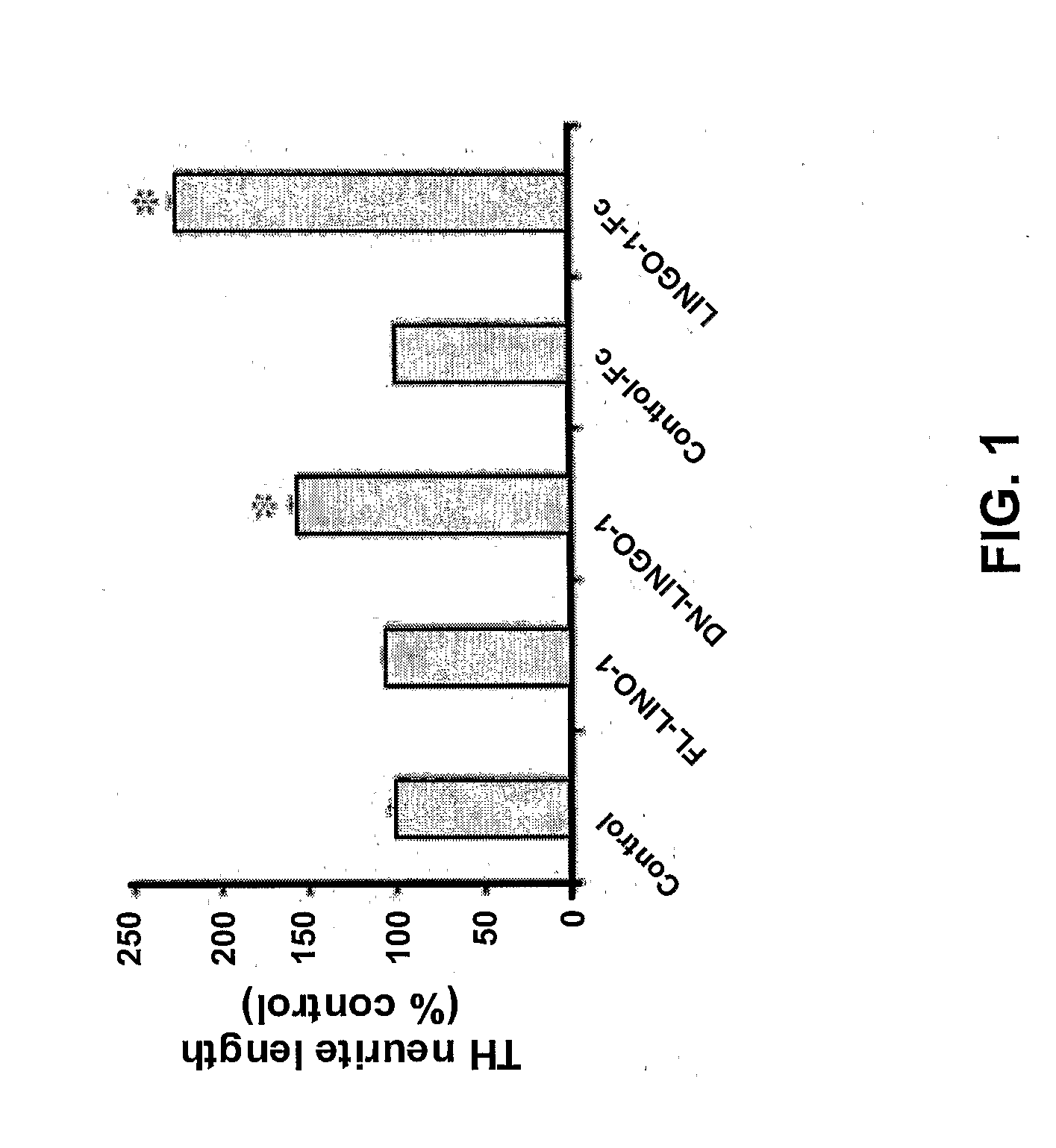
InactiveUS20090246189A1Promote regenerationPromoting outgrowthOrganic active ingredientsBiocideNeuronal degenerationDopamine
The present invention relates generally to methods for promoting regeneration, outgrowth and survival of dopaminergic neurons comprising contacting said dopaminergic neurons with an effective amount of a composition comprising an Sp35 antagonist. Additionally, the invention is related generally to methods of treating various diseases, disorders or injuries associated with dopaminergic neuronal degeneration or death by administration of an Sp35 antagonist.
Owner:THE MCLEAN HOSPITAL CORP +1
Therapeutic treatment for the metabolic syndrome, type2 diabetes, obesity, or prediabetes
InactiveUS20050054734A1Raise the ratioOrganic active ingredientsBiocideNervous systemNoradrenergic neurons
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic neuronal activity within the hypothalamus of the central nervous system of the patient. In another aspect, the present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of: administering to a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes a pharmaceutical composition comprising (1) at least one compound that stimulates an increase in central dopaminergic neuronal activity level in the subject, and (2) at least one compound that stimulates a decrease in central noradrenergic neuronal activity level in the subject. The present invention is also directed to pharmaceutical compositions that include the above compounds and a pharmaceutically acceptable carrier.
Owner:CINCOTTA ANTHONY H
Therapeutic Treatment for Metabolic Syndrome, Type 2 Diabetes, Obesity, or Prediabetes
InactiveUS20130274246A1Increases central neuronal dopamine activityPrevent arteriosclerosisBiocideMetabolism disorderAdrenergicPrediabetes
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic and / or serotonin neuronal activity within the central nervous system and particularly the hypothalamus of the central nervous system of the patient.
Owner:VEROSCI
Method for differentiating human neural stem cells into dopaminergic neuron by in-vitro directional induction
ActiveCN104031882AAvoid cross contaminationImprove securityNervous system cellsDiseaseCryopreservation
The invention discloses a method for differentiating human neural stem cells into dopaminergic neuron by in-vitro directional induction. The method comprises the following steps of adherent culture of human neural stem cells, induction of dopaminergic neuron precursor cells, directional induction of dopaminergic neuron and cryopreservation and anabiosis of the human neural stem cells and the dopaminergic neuron precursor cells. By virtue of the steps of the method disclosed by the invention, abundant dopaminergic neuron can be obtained in vitro; compared with the plurality of dopaminergic neuron production ways at present, the method disclosed by the invention has characteristics of low cost, high safety, high production efficiency and strong operability. The in-vitro dopaminergic neuron production way can provide a novel idea for applying dopaminergic neuron to clinical transplant treatment of the Parkinson disease.
Owner:SHANGHAI ANGECON BIOTECH
Derivation of terminally differentiated dopaminergic neurons from human embryonic stem cells
InactiveUS7674620B2Simple methodIncrease percentageNervous disorderCulture processDiseaseNeurulation
The present disclosure is directed to improved methods for efficiently producing neuroprogenitor cells and differentiated neural cells such as dopaminergic neurons and serotonergic neurons from pluripotent stem cells, for example human embryonic stem cells. Using the disclosed methods, cell populations containing a high proportion of cells positive for tyrosine hydroxylase, a specific marker for dopaminergic neurons, have been isolated. The neuroprogenitor cells and terminally differentiated cells of the present disclosure can be generated in large quantities, and therefore may serve as an excellent source for cell replacement therapy in neurological disorders such as Parkinson's disease.
Owner:RELIANCE LIFE SCI PVT
Induction and high-yield preparative purification of mesencephalic dopaminergic neuronal progenitor cells and dopaminergic neurons from human embryonic stem cells
InactiveUS20050196864A1Ameliorate motor behavior deficitLower Level RequirementsNervous system cellsMammal material medical ingredientsProgenitorDopamine
The present invention relates to an enriched or purified population of dopaminergic neuronal progenitor cells and an enriched or purified population of dopaminergic neurons. These enriched or purified populations are derived from a population of embryonic stem cells by inducing production of dopaminergic neuronal progenitor cells. A promoter or enhancer which functions only in dopaminergic neuronal progenitor cells is selected and a nucleic acid molecule encoding a marker protein under control of said promoter or enhancer is introduced into the induced population of embryonic stem cells. The dopaminergic neuronal progenitor cells are allowed to express the marker protein, and the cells expressing the marker protein are separated from the induced population of embryonic stem cells. As a result, an enriched or purified population of dopaminergic neuronal progenitor cells is isolated. Alternatively, the nucleic acid molecule encoding the marker protein under control of the promoter or enhancer is introduced into the population of human embryonic stem cells followed by induction of the population of embryonic stem cells.
Owner:CORNELL RES FOUNDATION INC
Dopaminergic neurons and proliferation-competent precursor cells for treating Parkinson's disease
InactiveUS20050042749A1Promote generationHigh proportionGenetically modified cellsMicrobiological testing/measurementProgenitorNervous system
This disclosure provides improved methods for obtaining populations of neural progenitor cells and differentiated neurons from pluripotent stem cells. The technology can be used to produce progenitors that proliferate through at least 40 doublings, while maintaining the ability to differentiate into a variety of different neural phenotypes. Cell populations have been obtained that contain a high proportion of cells staining for tyrosine hydroxylase, which is a feature of dopaminergic neurons. The neural progenitors and terminally differentiated neurons of this invention can be generated in large quantities for use in drug screening and the treatment of clinically important neurological disorders, such as Parkinson's disease.
Owner:ASTERIAS BIOTHERAPEUTICS INC
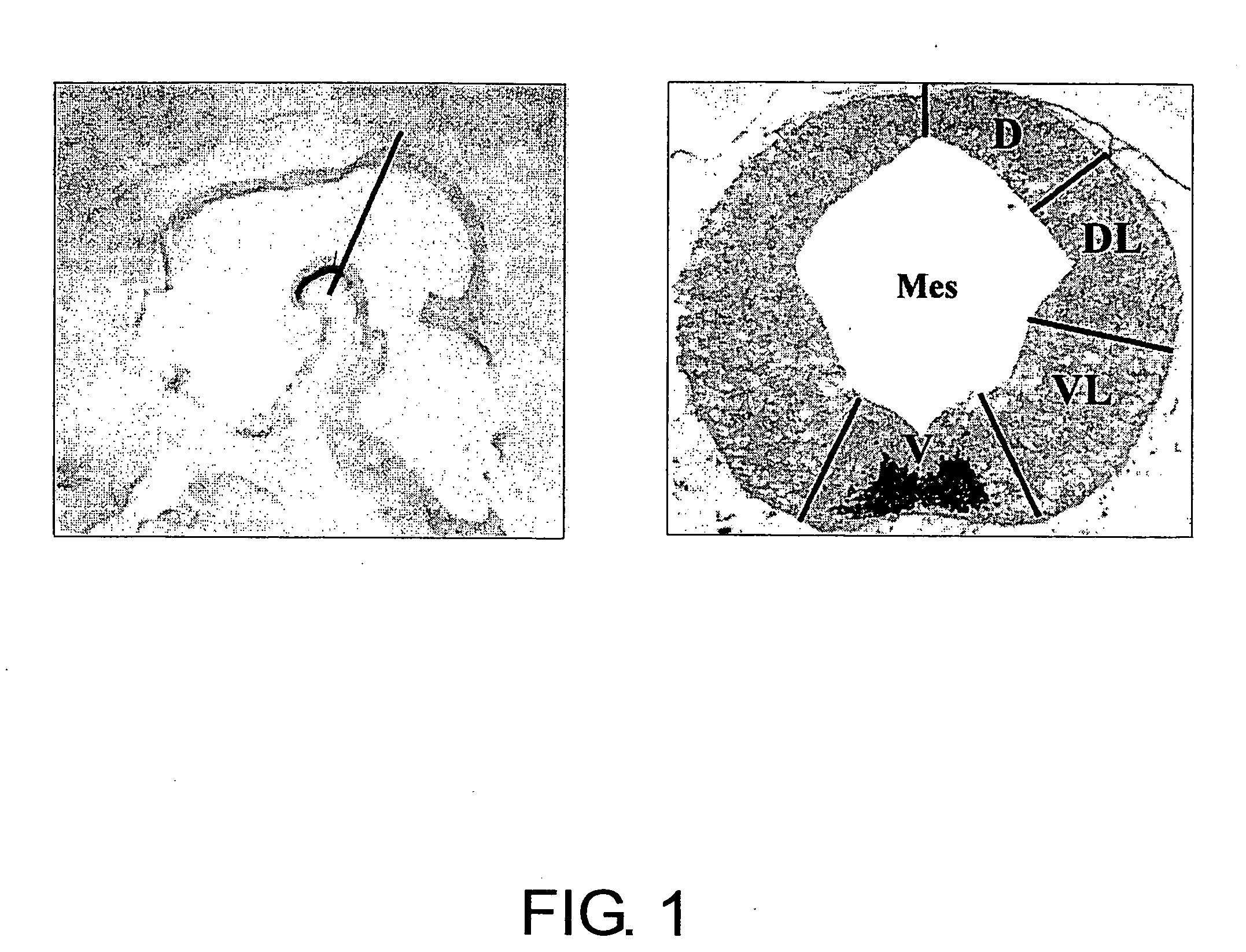
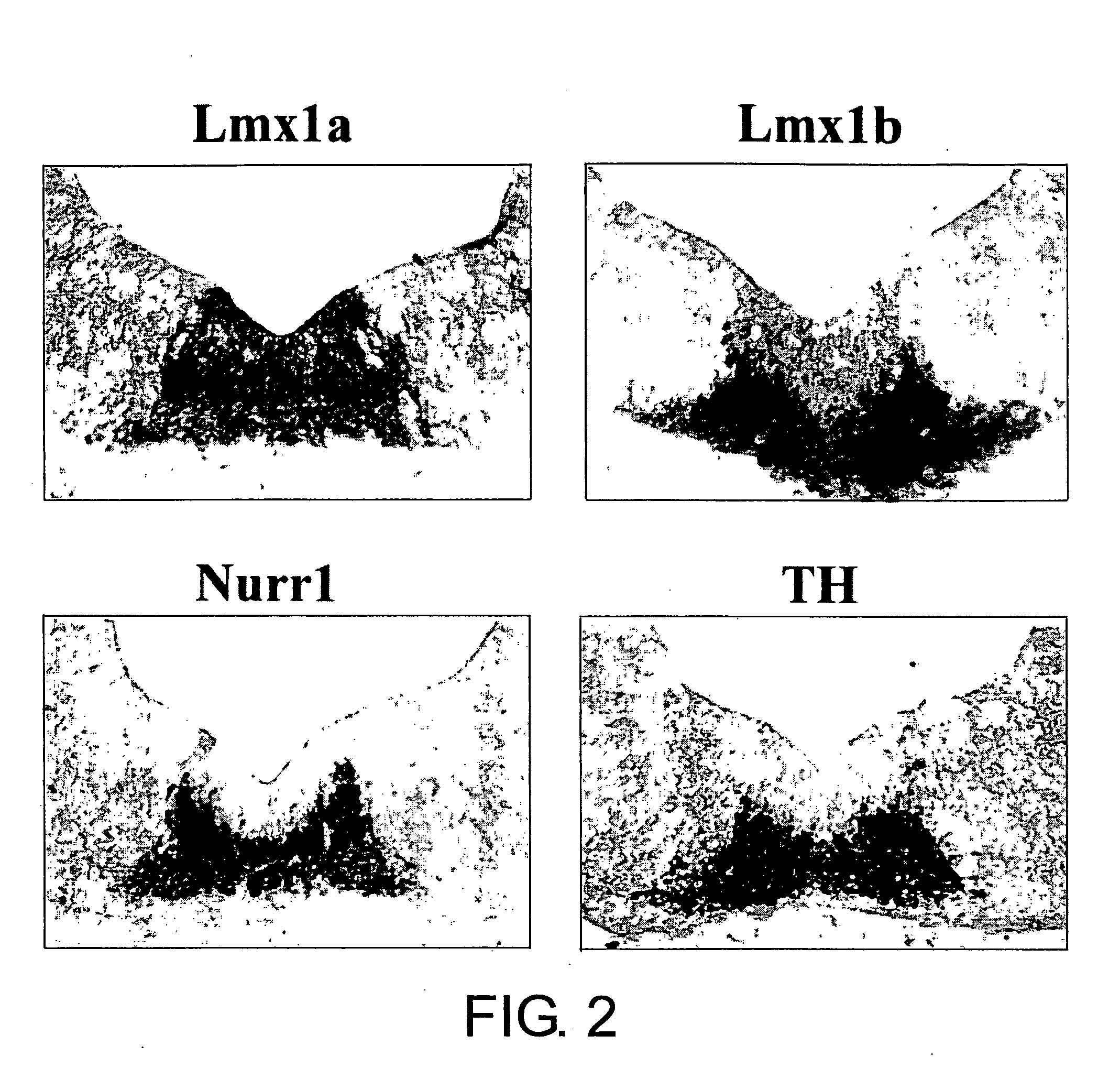
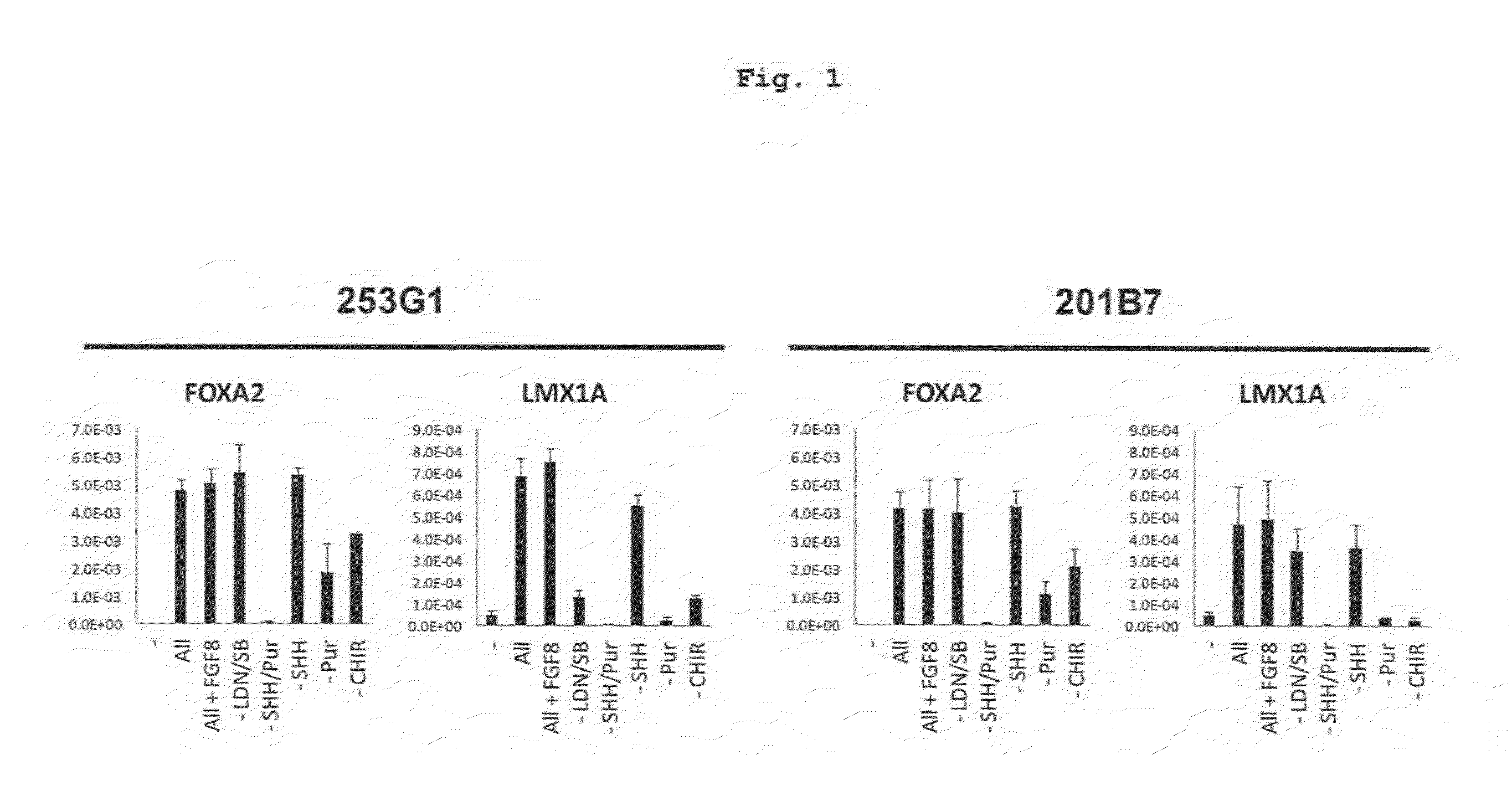
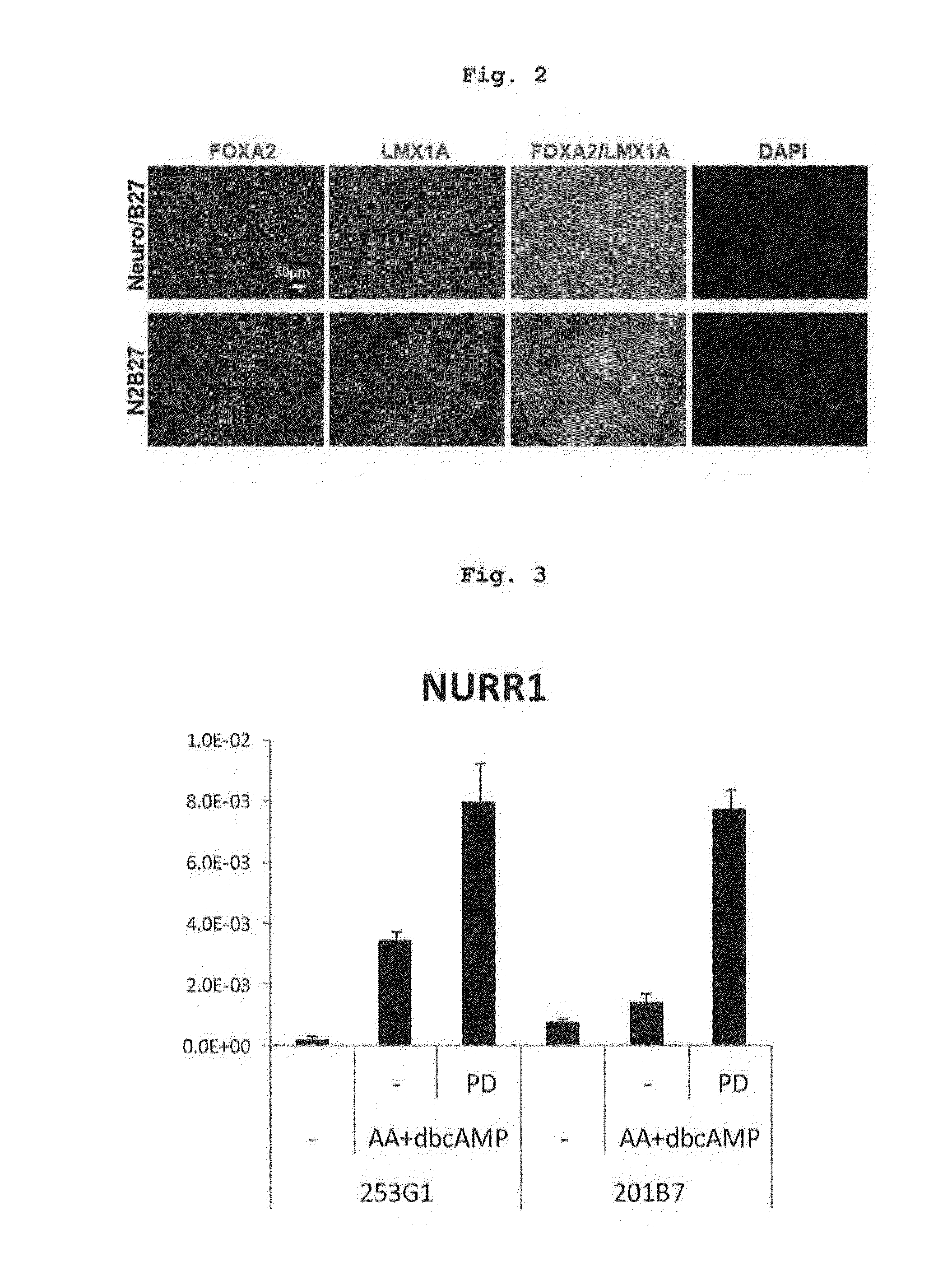
Specific Marker Lmx1a on Dopaminergic Neurons
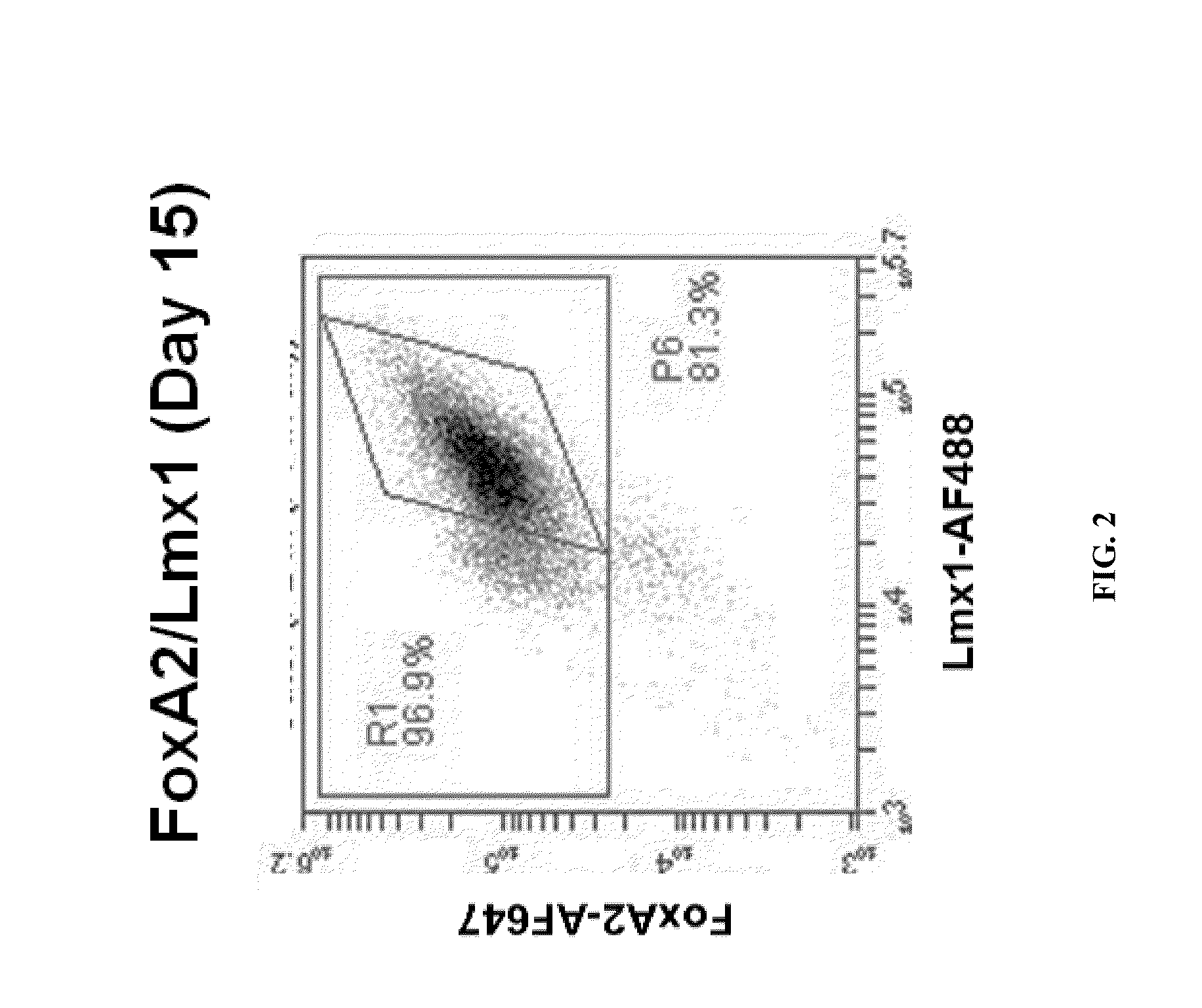
ActiveUS20070254281A1Effective markerMaintain expressionMicrobiological testing/measurementDisease diagnosisProgenitorLMX1A gene
The present invention identified Lmx1a genes, which are expressed in dopaminergic neurons at all differentiation stages, from proliferating dopaminergic neuron progenitor cells before cell cycle exit to cells after cell cycle exit. Lmx1a expression in cells can be used as an indicator when selecting cells suitable for transplantation therapy for neurodegenerative diseases such as Parkinson's disease, and is useful as a marker for screening agents involved in the induction of dopaminergic neuron differentiation.
Owner:EISIA R&D MANAGEMENT CO LTD
Human mesencephalon cell lines and methods of use therefor
Conditionally-immortalized human mesencephalon cell lines are provided. Such cell lines, which may be clonal, may be used to generate neurons, including dopaminergic neurons. The cell lines and / or differentiated cells may be used for the development of therapeutic agents to prevent and treat a variety of neurological diseases such as Parkinson's disease. The cell lines and / or differentiated cells may also be used in assays and for the general study of mesencephalon cell development and differentiation.
Owner:SIGNAL PHARM INC
Method for producing dopaminergic neurons
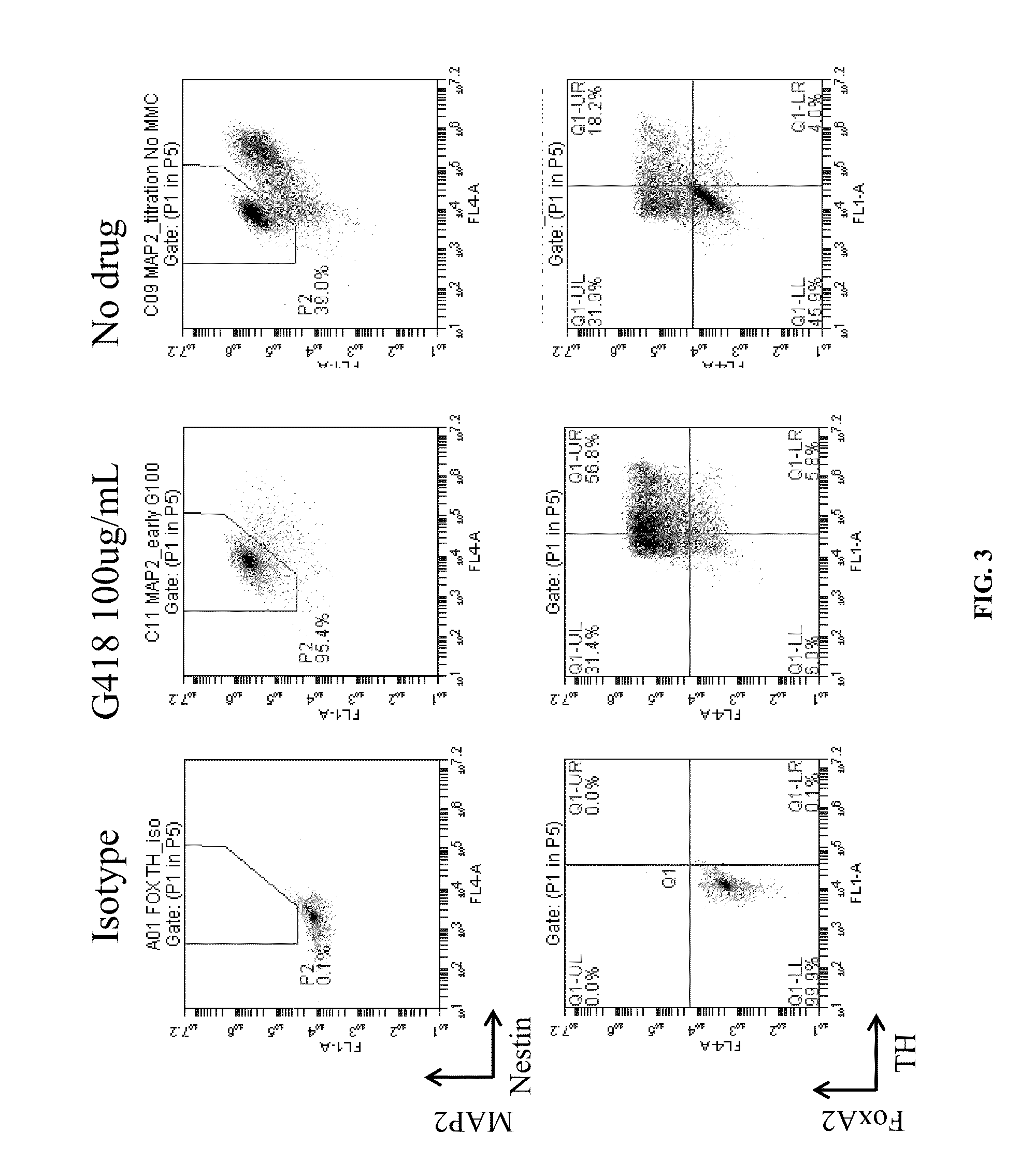
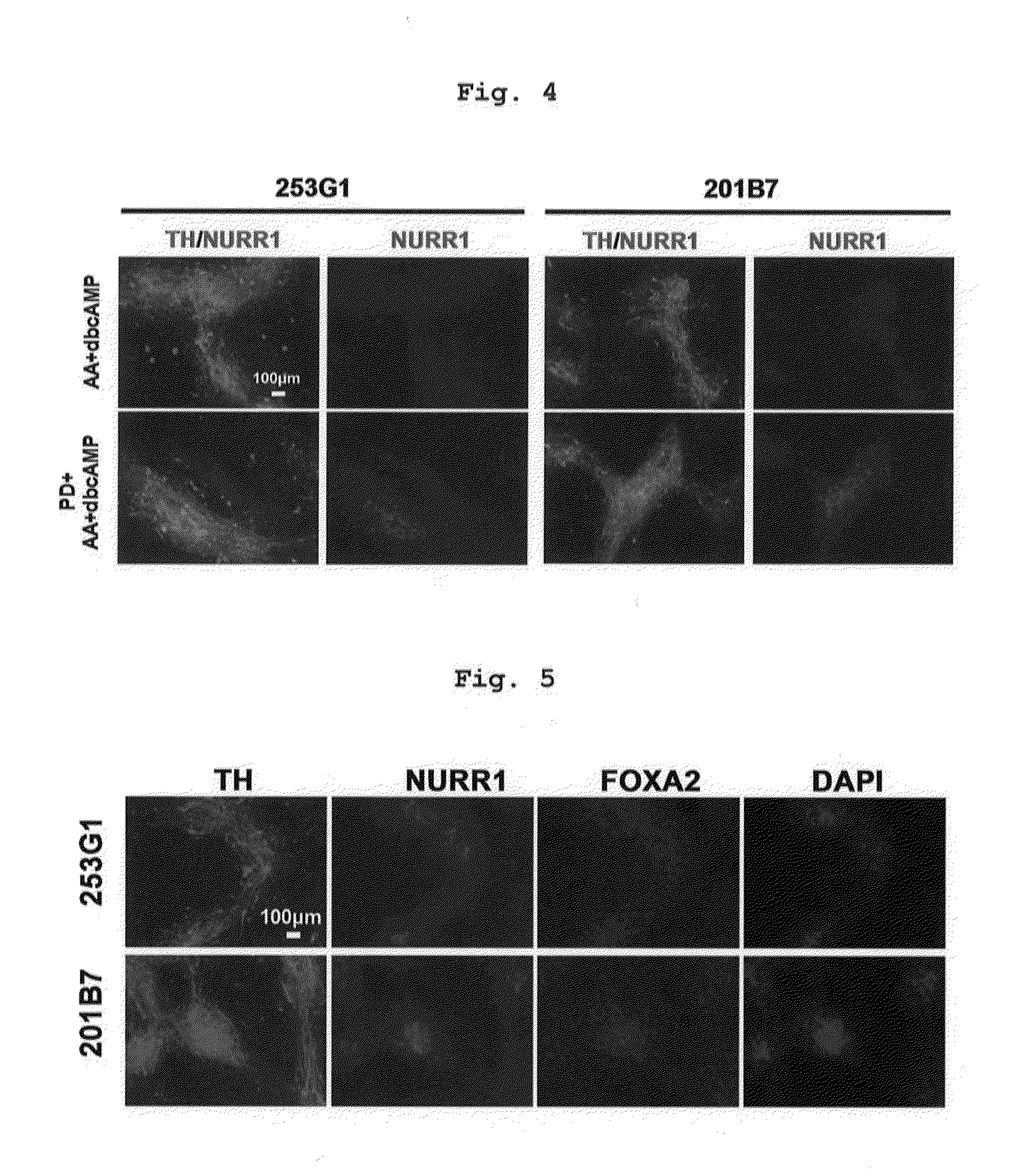
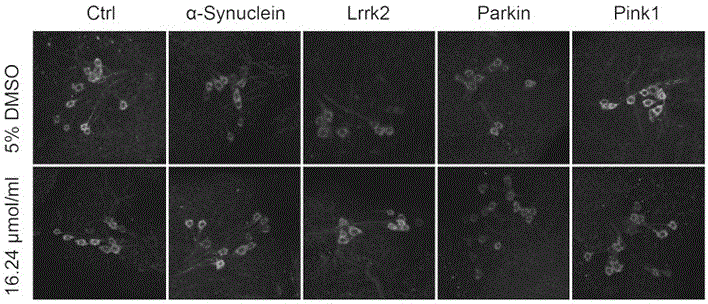
ActiveUS20160177260A1High-quality dopaminergicEfficient productionBiocideNervous disorderProgenitorMEK inhibitor
The present invention provides a method of more efficiently producing a high-quality dopaminergic neuron from neural progenitor cells, specifically, a production method of a dopaminergic neuron including a step of culturing neural progenitor cells in a medium containing (i) a cAMP analogue and (ii) a MEK inhibitor. Moreover, the present invention also provides a medicament containing a dopaminergic neuron obtained by the method, and a reagent and a kit to be used for the method.
Owner:TAKEDA PHARMA CO LTD
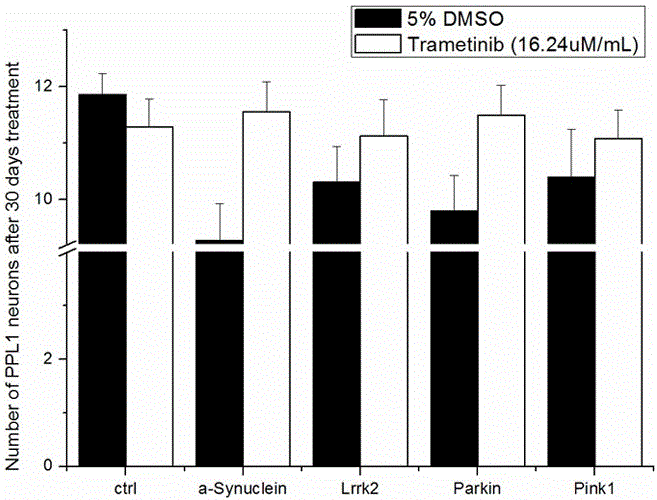
Application of trametinib in preparation of drug for treating Parkinson's disease
ActiveCN105816461ADelay degenerative deathGood curative effectOrganic active ingredientsNervous disorderCurative effectTherapeutic effect
The invention belongs to the technical field of medicine, and discloses application of trametinib in preparation of a drug for treating Parkinson's disease, wherein trametinib is used for preparing the drug to delay the degeneration of dopaminergic neuron. The obtained product can effectively delay the degenerative death of dopaminergic neuron, to treat Parkinson's disease fundamentally; the drug can be taken orally without injection to achieve the treatment effect, has excellent curative effect for Parkinson's disease caused by a variety of reasons, and has important significance for the treatment and curing of Parkinson's disease.
Owner:FUZHOU UNIV
Gene expressed specifically in dopamine-producing neuron precursor cells after termination of division
InactiveUS20060239978A1Increase speedHigh sensitivityFungiBacteriaNeuro-degenerative diseaseNovel gene
A novel gene 65B13 expressed specifically and transiently in dopaminergic neuron precursor cells immediately after cell cycle exit was obtained by the present invention. The cellular expression of 65B13 can be used as an index to select cells that are suitable in terms of their safety, survival rate, and network formation ability, for transplant therapy of neurodegenerative diseases such as Parkinson's disease.
Owner:EISIA R&D MANAGEMENT CO LTD
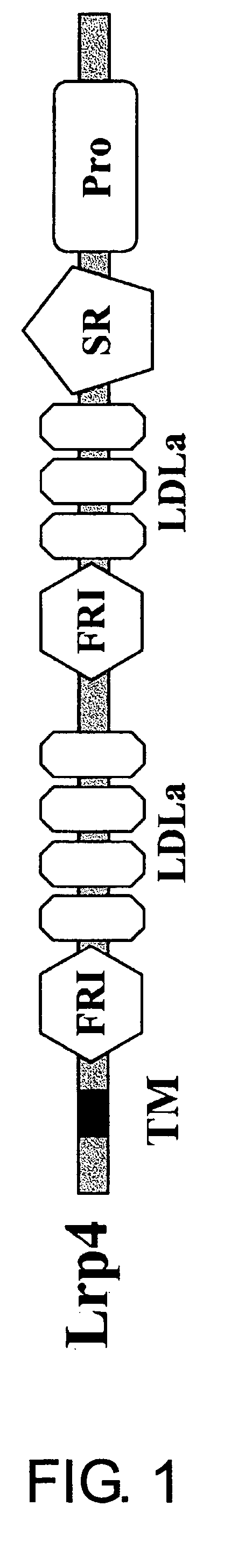
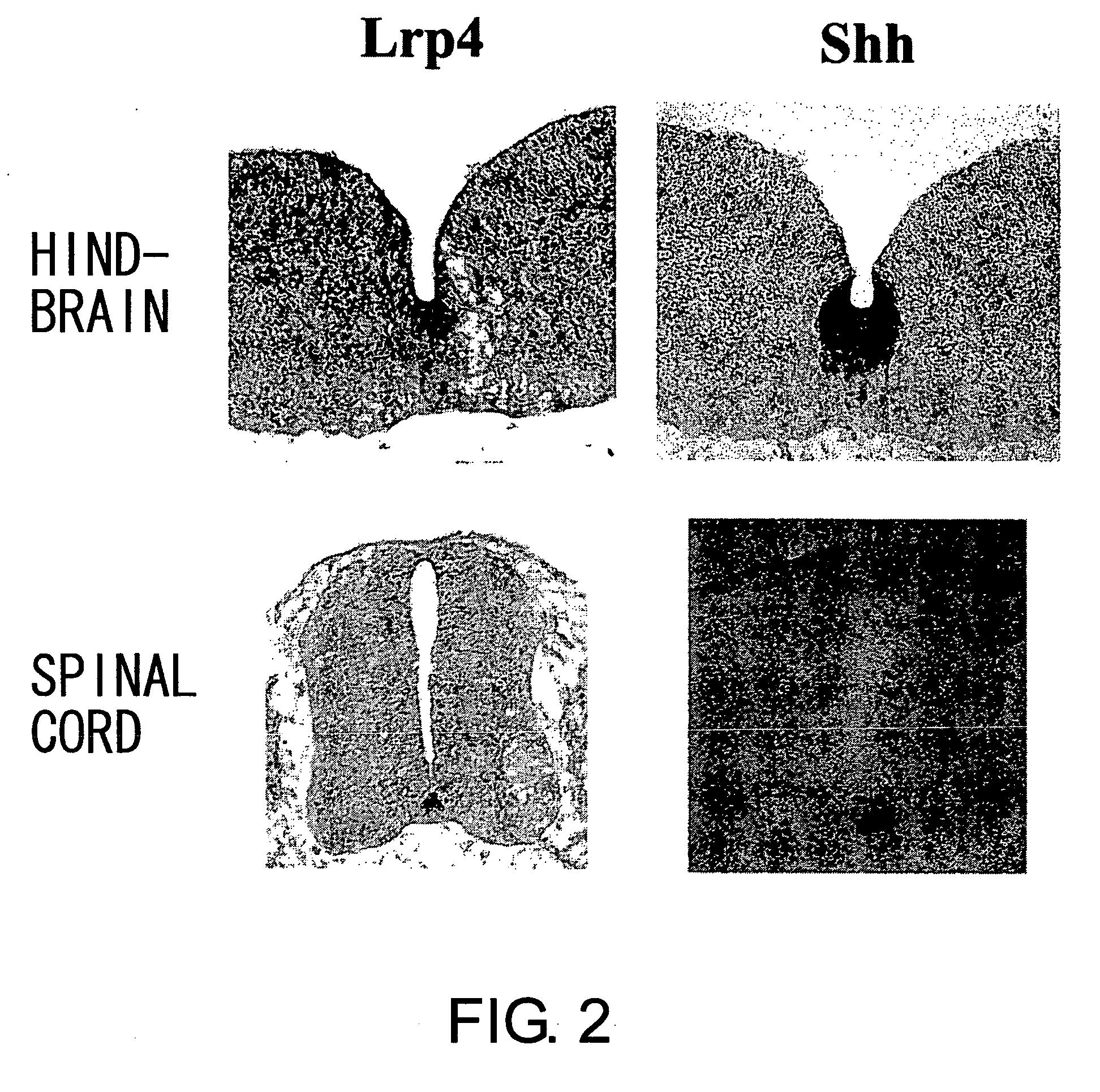
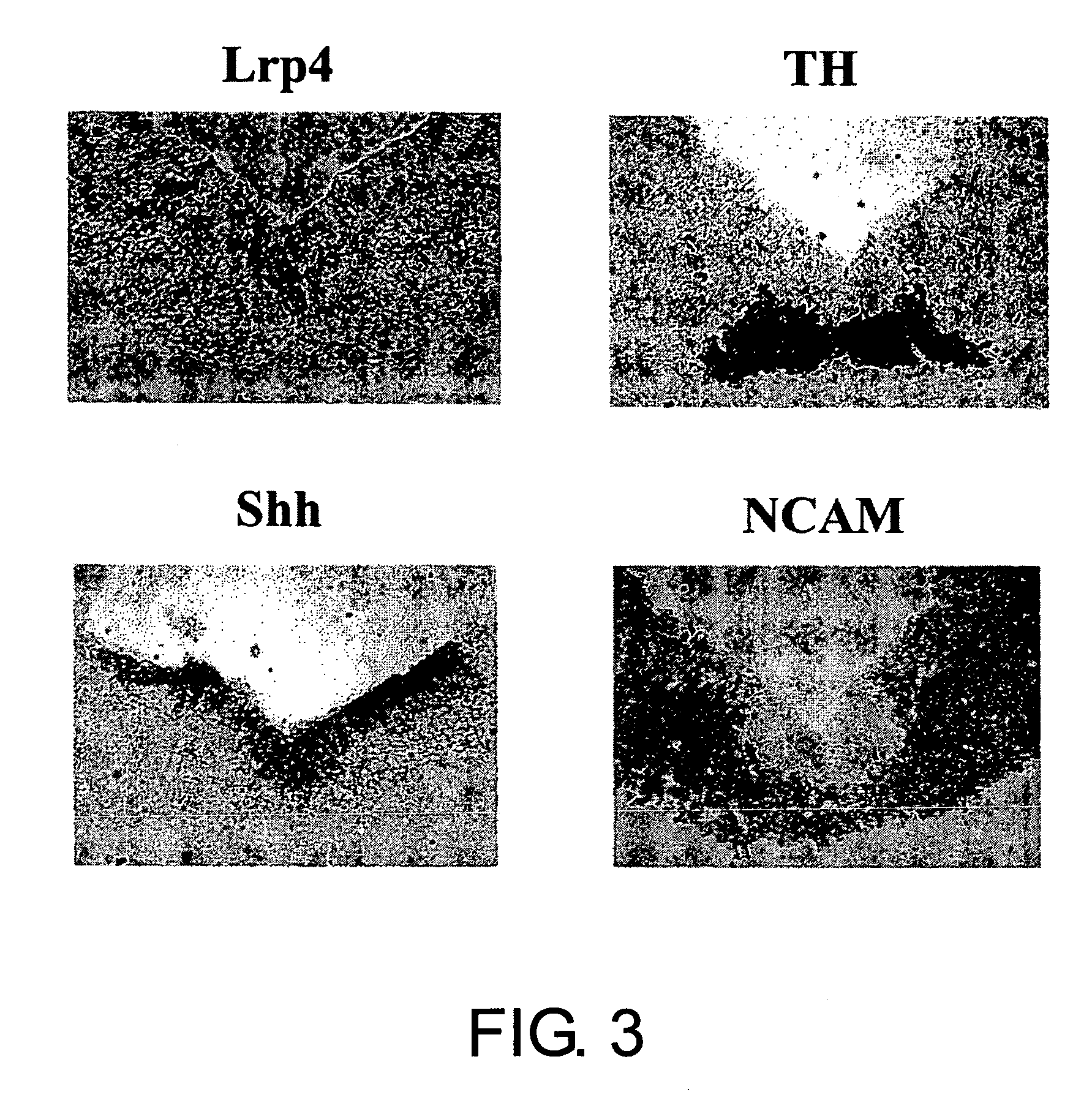
Lrp4/corin dopamine-producing neuron proliferation precursor cell marker
InactiveUS20060240432A1Increase speedHigh sensitivityNervous disorderMicrobiological testing/measurementProgenitorTherapeutic effect
In neuron transplantation therapy, in terms of safety, it is preferable to use a cell population consisting only of a desired type of cells, and to use postmitotic neurons in consideration to avoid the risk of tumorigenesis. Moreover, greater therapeutic effects would be expected through the use of earlier progenitor cells in consideration of post-transplantation viability, proper network formation ability, and such. According to the present invention, Lrp4, a gene that is specifically expressed in dopaminergic neuron proliferative progenitor cells prior to cell cycle exit, was identified. The use of Lrp4 expression in cells as an index allows for the isolation. of cells suitable for transplantation therapy of neurodegenerative diseases such as Parkinson's disease in terms of safety, survival rate, and network formation ability.
Owner:EISIA R&D MANAGEMENT CO LTD
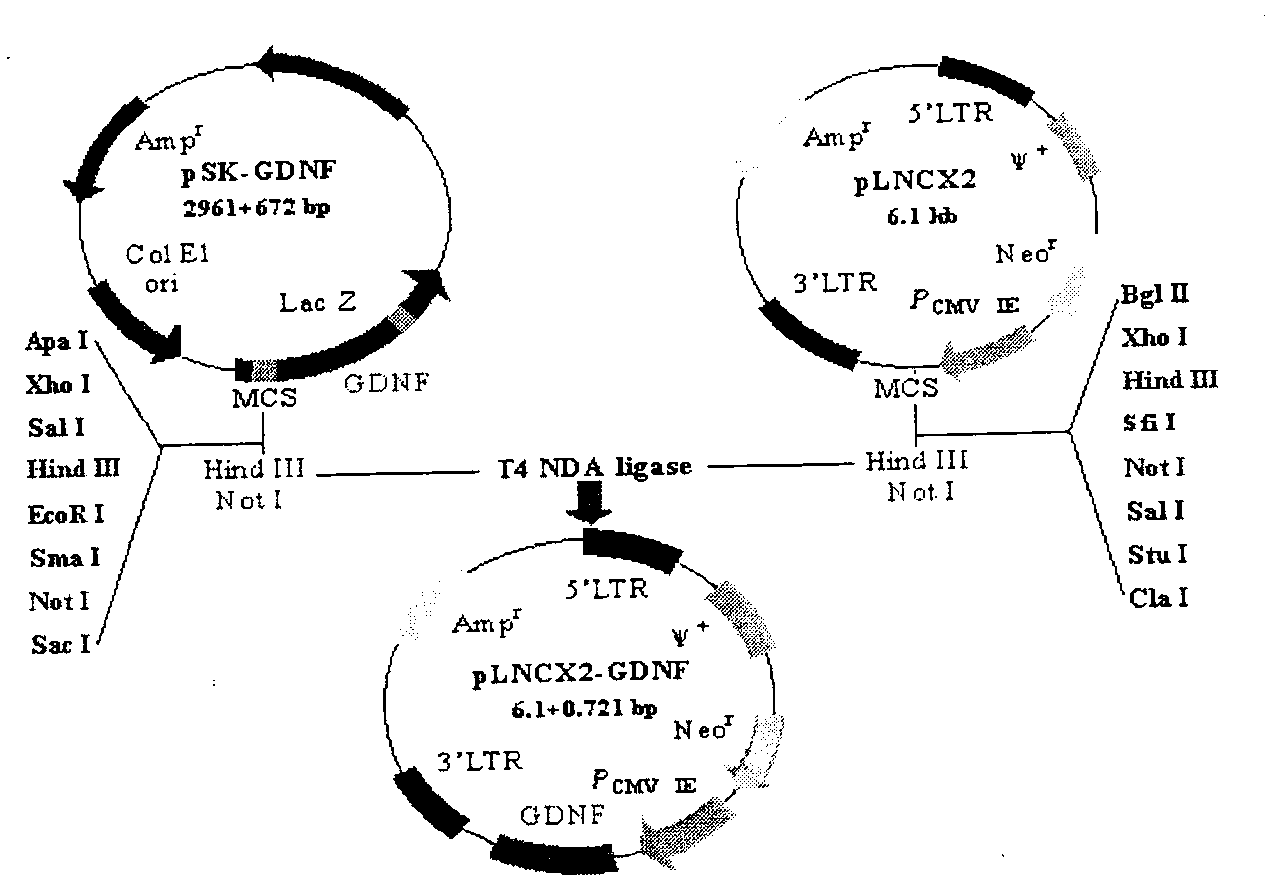
Construction method of glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell
InactiveCN103215225AViruses/bacteriophagesBiological testingGlial cell line-derived neurotrophic factorNeuronal degeneration
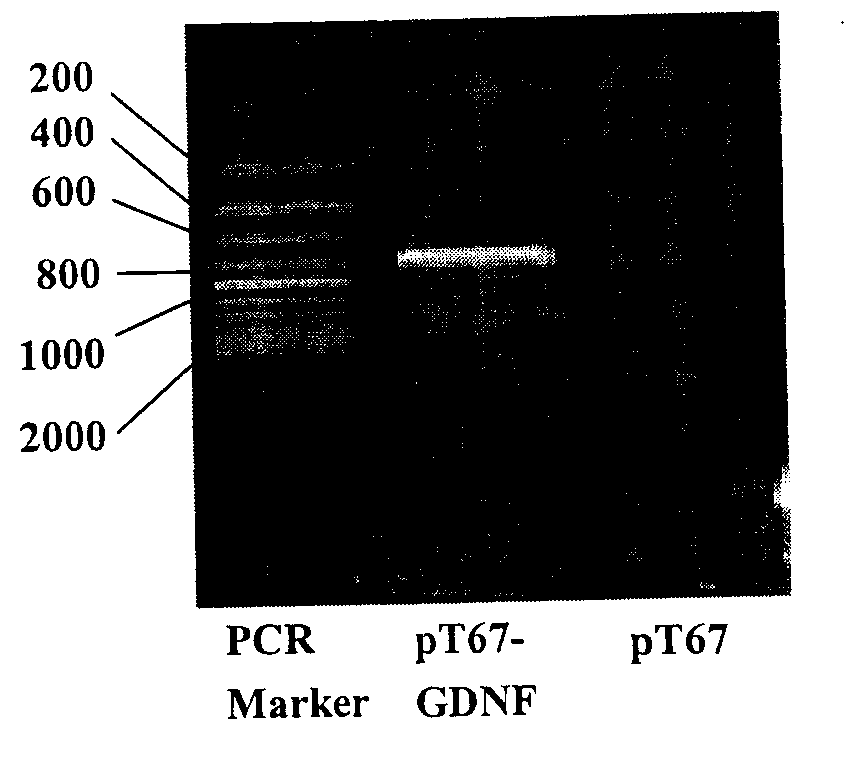
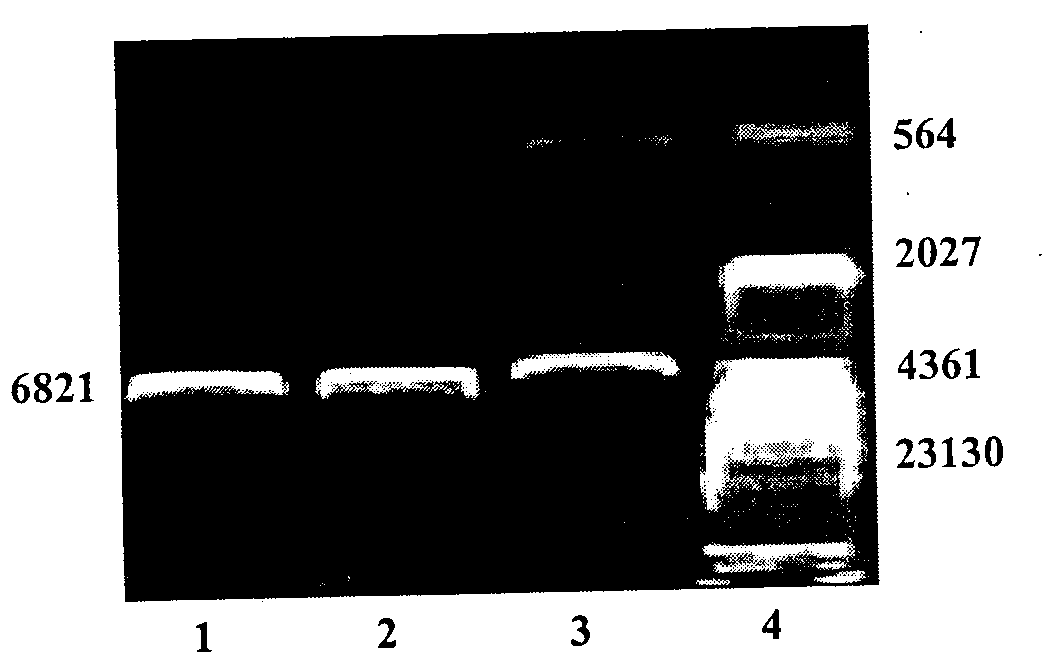
The invention relates to a biotechnology and especially relates to a construction method of a glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell. The construction method comprises the following steps of 1, plasmid transformation, 2, plasmid digestion purification, 3, target gene and vector cloning, 4, recombinant plasmid packaging, 5, recombinant plasmid identification, 6, virus titer determination, 7, stem cell culture, 8, virus infection of the stem cells, 9, transgenic stem cell identification, 10, transgenic stem cell target-protein content determination, and 11, transgenic stem cell biological-activity detection. The glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell has strong effects of promoting survival of dopaminergic neurons and retains characteristics of the original neural stem cell. The glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell has good effects of treating motor neuron damage diseases and neuronal degeneration diseases.
Owner:孙勇
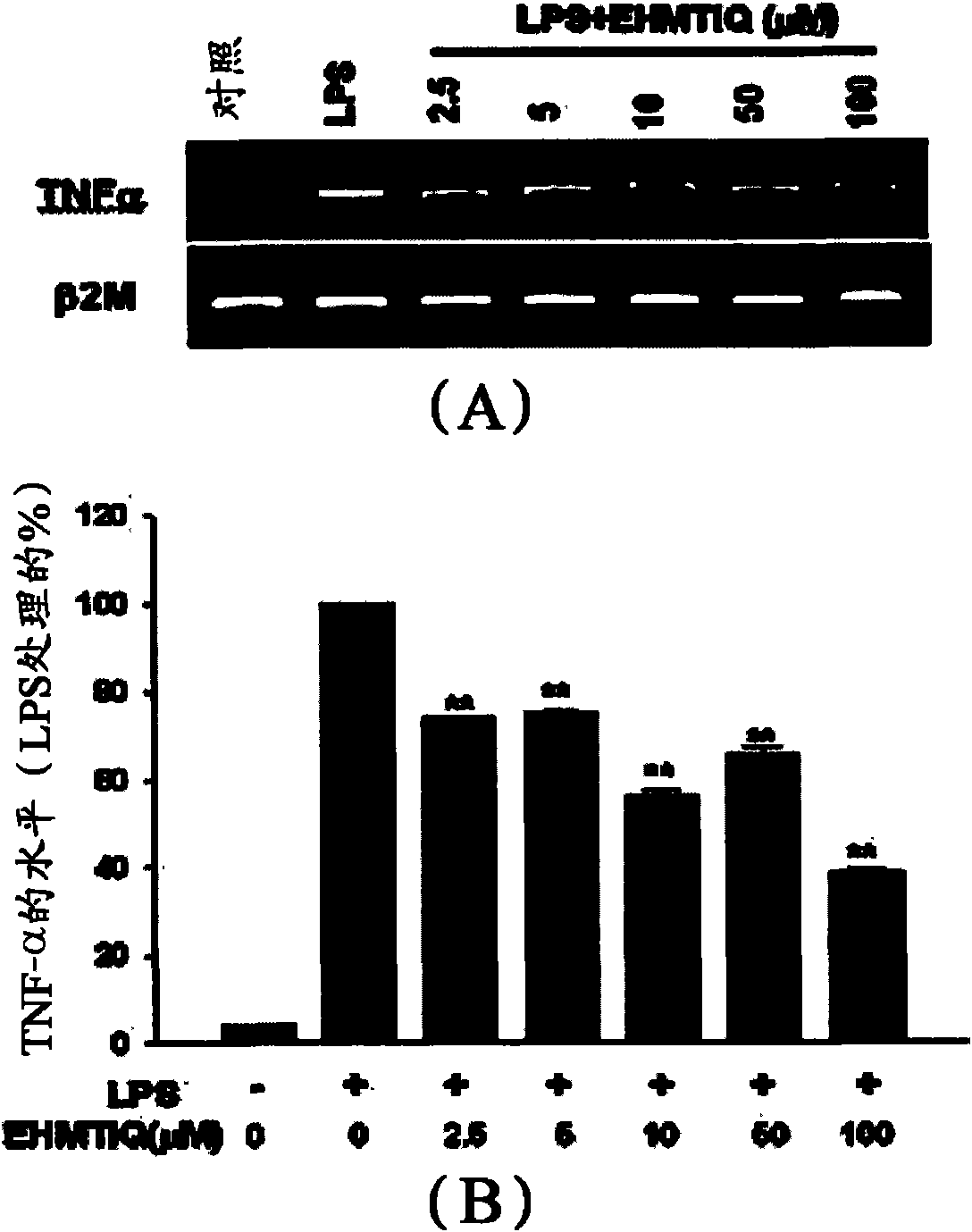
1,2,3,4-tetrahydroisoquinoline derivatives having effects of preventing and treating degenerative and inflammatory diseases
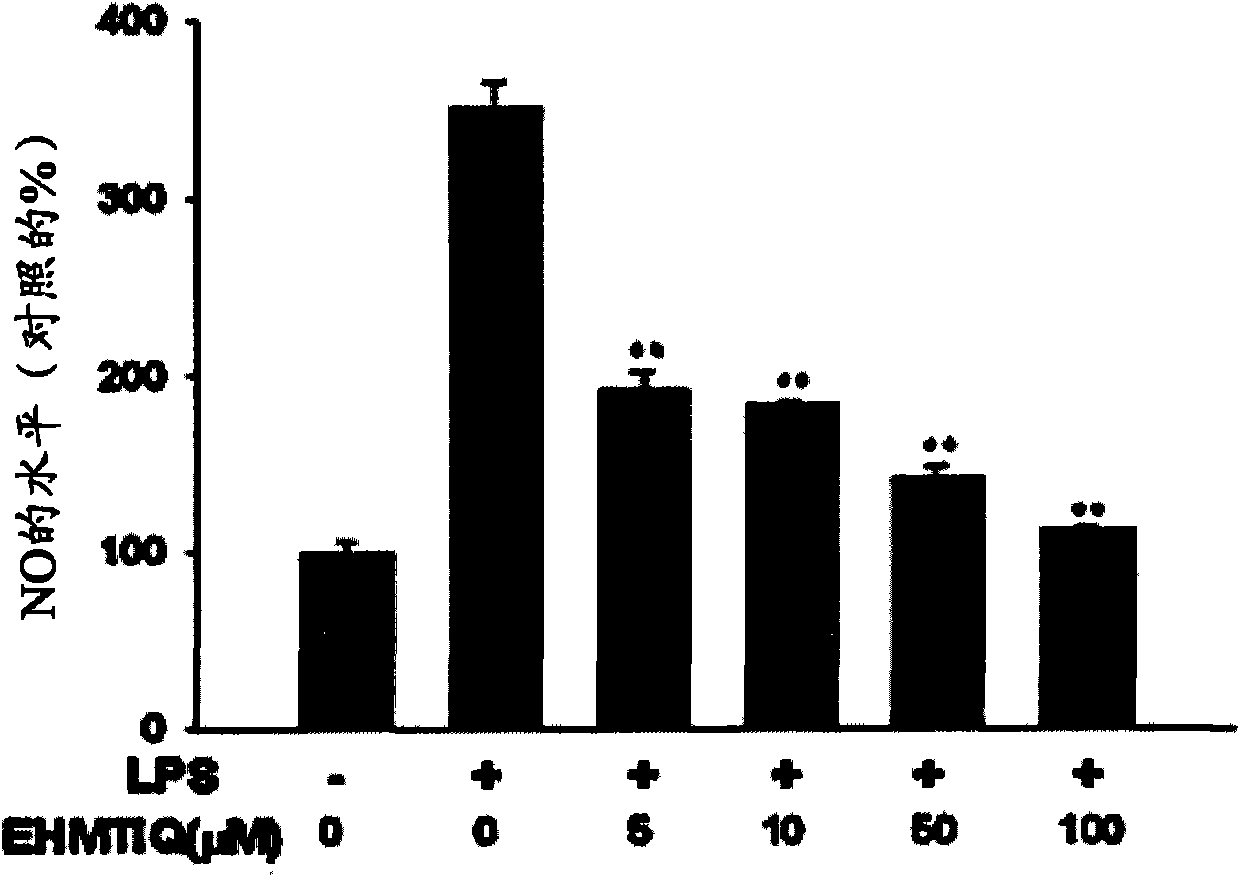
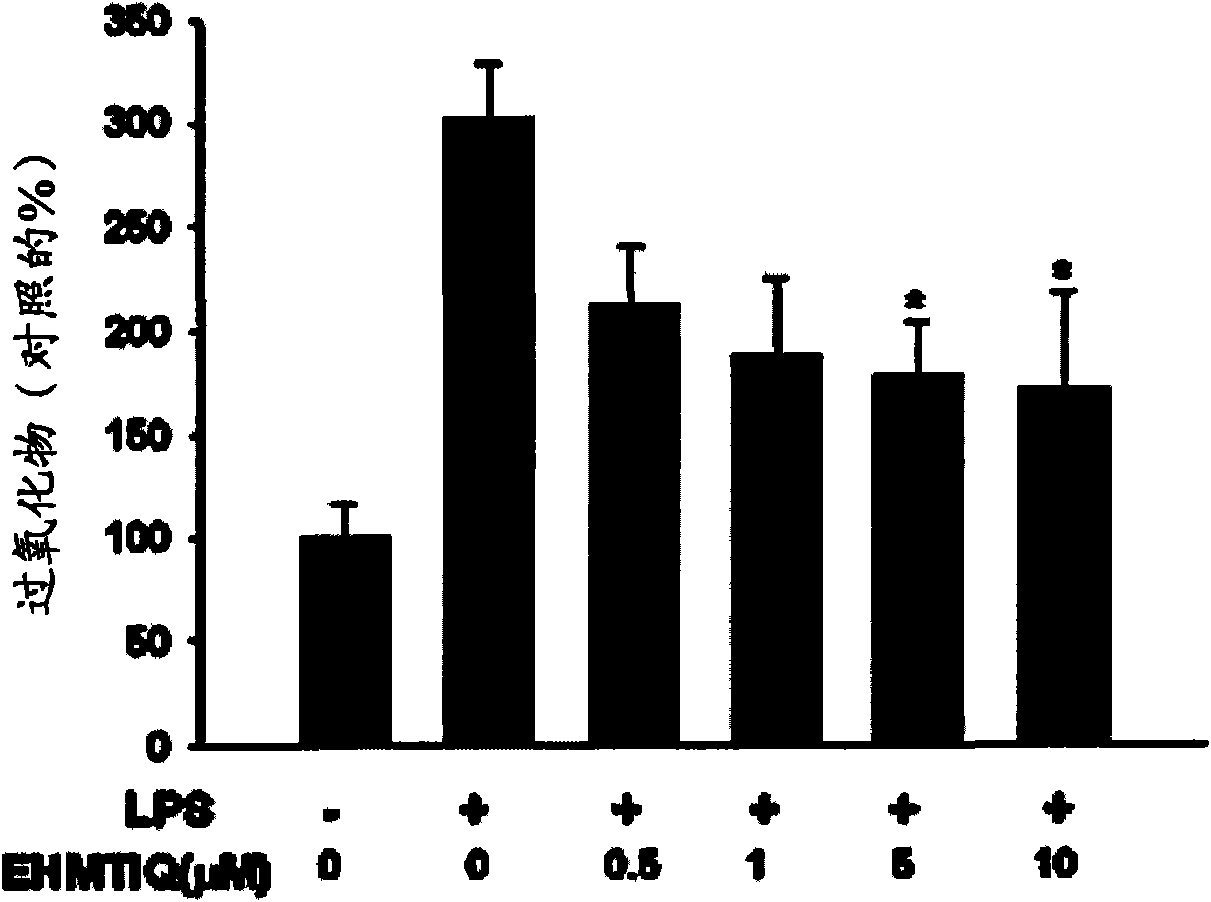
InactiveCN101553229AInhibit expressionInhibit overproductionOrganic active ingredientsNervous disorderActivated microglial cellSynthesis methods
Provided are 7-hydroxy-6-methoxy-1,2,3,4-tetrahydroisoquinoline derivatives and synthesis methods thereof. The compounds significantly inhibit the production of nitrogen monoxide (NO) and superoxide in an activated microglial cell and expressions of TNF-alpha, IL- 1beta inducive NO synthase and cyclooxygenase-2 genes. They also prevent NF-kB shift to a nucleus, decrease reactive oxygen species (ROS), inhibit expression of GTP cyclohydrolase I gene and over-production of tetrahydrobiopterin (BH 4 ), and protect dopaminergic neurons from injury due to activated microglial cells. Consequently, the compounds are effective in treating inflammatory and neurodegenerative diseases.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
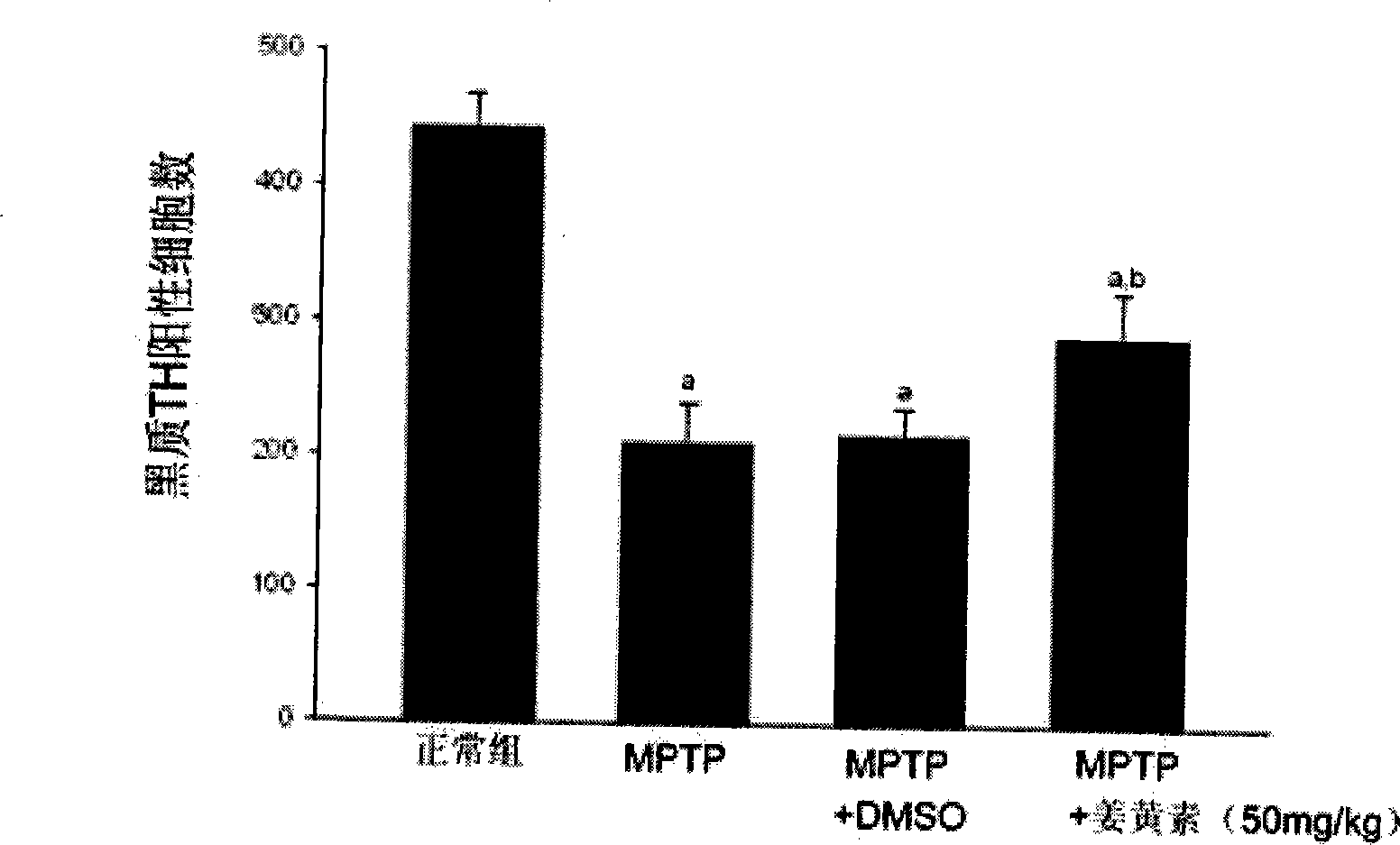
Application of turmeric native
InactiveCN101366709AAvoid damageNervous disorderKetone active ingredientsDiseaseDopamine biosynthesis
The invention discloses novel application of a medicine - curcumin, namely application of the curcumin in preventing and treating or treating the Parkinson disease. After the curcumin acts on an MPTP-constructed Parkinson disease mouse model, the curcumin can effectively promote expression of tyrosine hydroxylase in substantia nigra, lighten damage of sneurotoxin on the tyrosine hydroxylase in substantia nigra, reduces damage of dopaminergic neurons, promote biosynthesis of damaged dopamine and promote restoration and growth of damaged dopaminergic neurons; the curcumin can also inhibit astrocytes to secrete gelatinous fiber proteins, reduce stimulus of the neurotoxin on the astrocytes and lighten phlegmonosis of a brain tissue; moreover, the curcumin can inhibit expression of induction type nitrous oxide synthase, lighten induction of the neurotoxin on the induction type nitrous oxide synthase and inhibit reinforcement of intracephalic oxidative stress reaction. Therefore, the curcumin can effectively reduce damage of the dopaminergic neurons, thereby having significant potential value in developing medicines for preventing and treating and / or treating the Parkinson disease.
Owner:RUIJIN HOSPITAL ATTACHED TO SHANGHAI NO 2 MEDICALUNIV +1
Uses of interleukin-22(il-22) in treating and preventing nerve damage diseases or neurodegenerative diseases
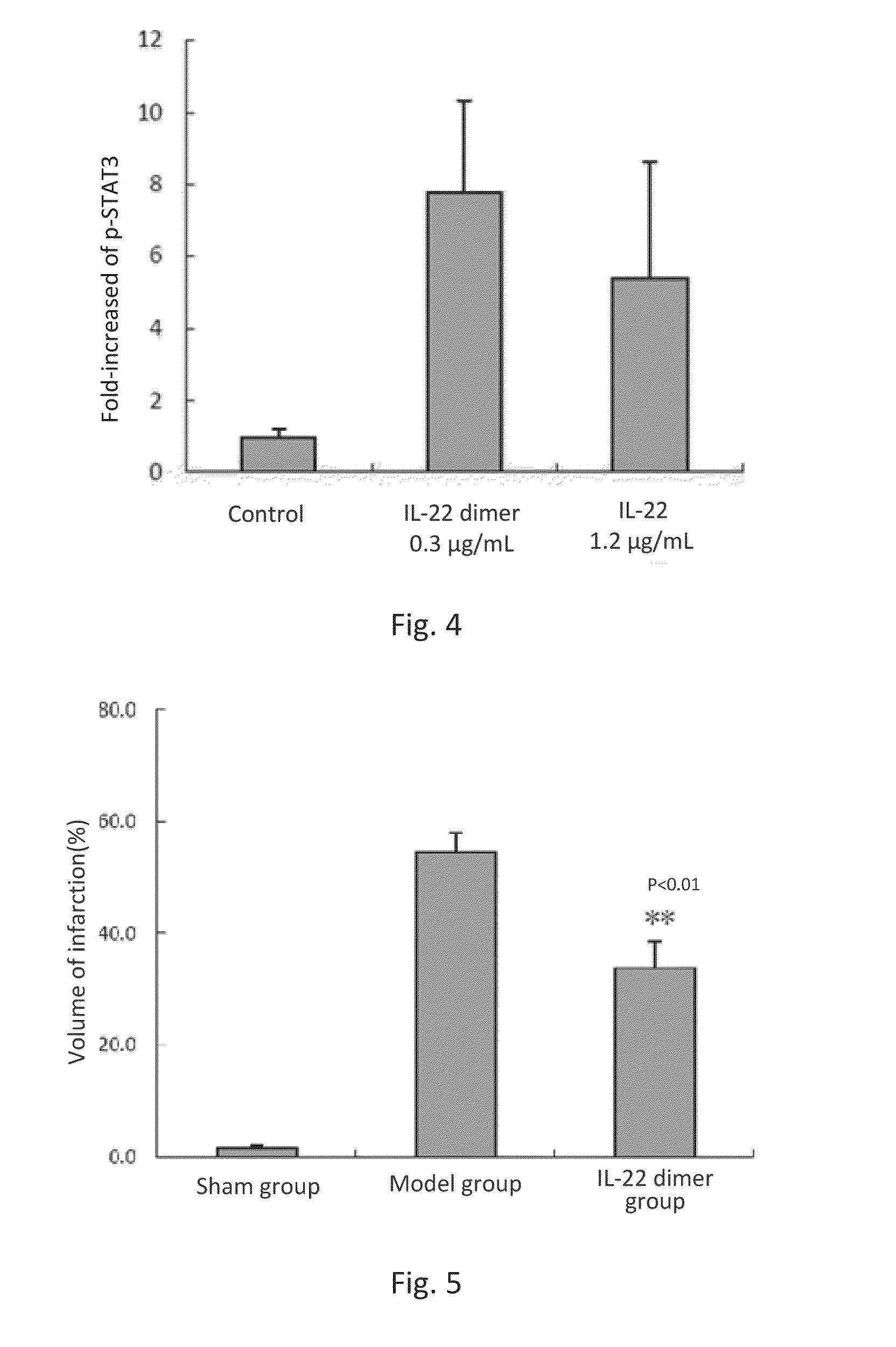
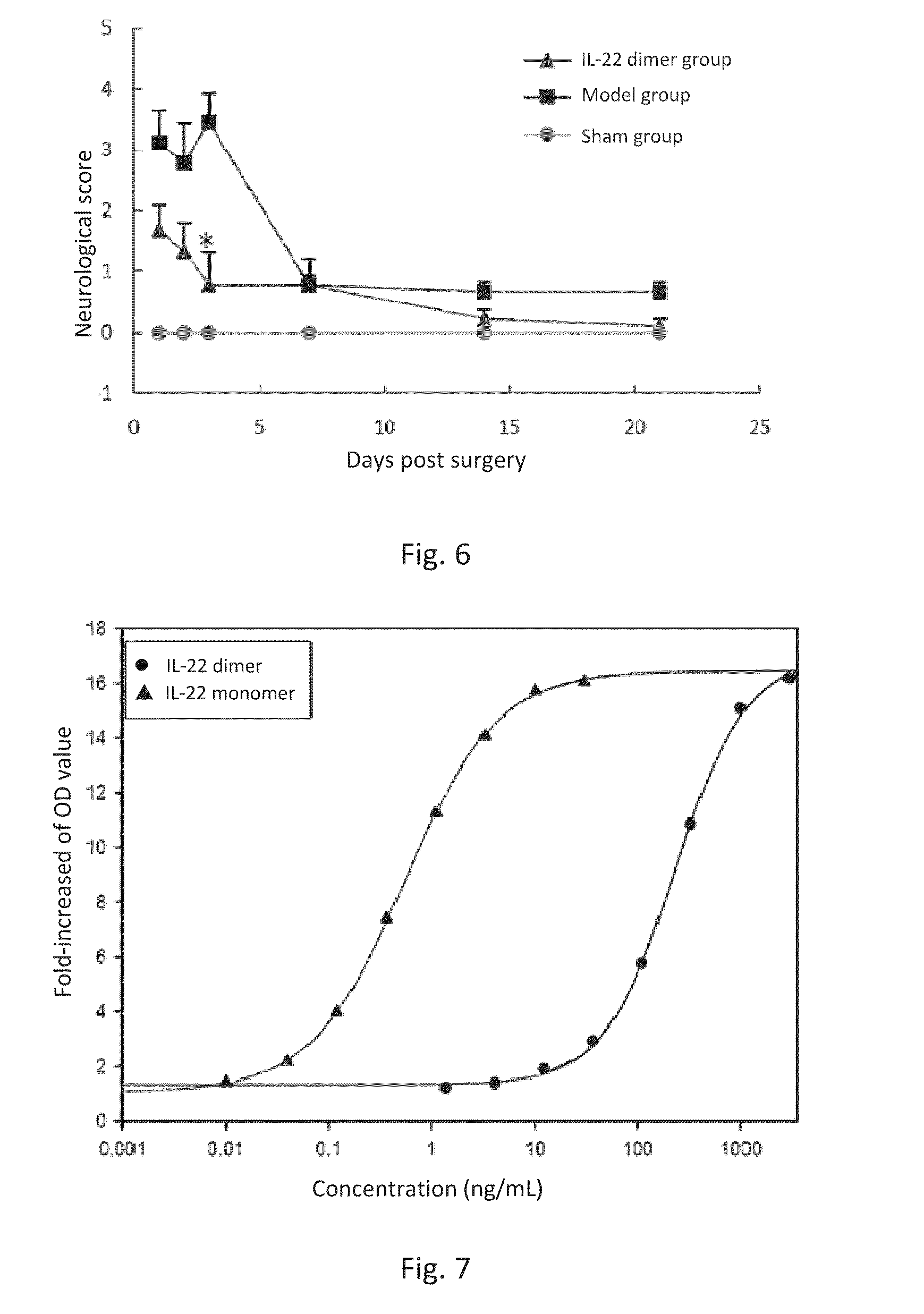
ActiveUS20150147293A1Enabling recoveryFunction increaseNervous disorderPeptide/protein ingredientsIn vivoDisease cause
This invention discloses the uses of IL-22 in the treatment and prevention of a nerve damage disease or a neurodegenerative disease. In particular, the invention discloses the uses of IL-22 or IL-22 dimers as follows: (i) can protect neurons to recover the functions of injured neurons after ischemic nerve damage in animals in vivo, thus enabling effective treatment of nerve damage diseases, (ii) can significantly inhibit the loss of dopaminergic neurons in substantia nigra in PD model animal, enhance the functions of dopaminergic neurons, significantly reduce neuronal apoptosis in hippocampus, improve learning and memory capacity of AD model rats, and effectively prevent neuronal loss, thereby enabling more effective treatment of neurodegenerative diseases.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Method for inducing dopaminergic neuron progenitor cells
ActiveUS20160215260A1Improve survival rateEfficiently obtainedNervous disorderNervous system cellsProgenitorCell-Extracellular Matrix
The present invention provides a method for producing dopaminergic neuron progenitor cells from pluripotent stem cells, which method comprises the steps of: (i) performing adherent culture of pluripotent stem cells on an extracellular matrix in a medium containing a reagent(s) selected from the group consisting of BMP inhibitor, TGFβ inhibitor, SHH signal-stimulating agent, FGF8, and GSK3β inhibitor; (ii) collecting Corin- and / or Lrtm1-positive cells from the cells obtained in Step (i) using a substance which binds to Corin and / or a substance which binds to Lrtm1; and (iii) performing suspension culture of the cells obtained in Step (ii) in a medium containing a neurotrophic factor.
Owner:KYOTO UNIV +2
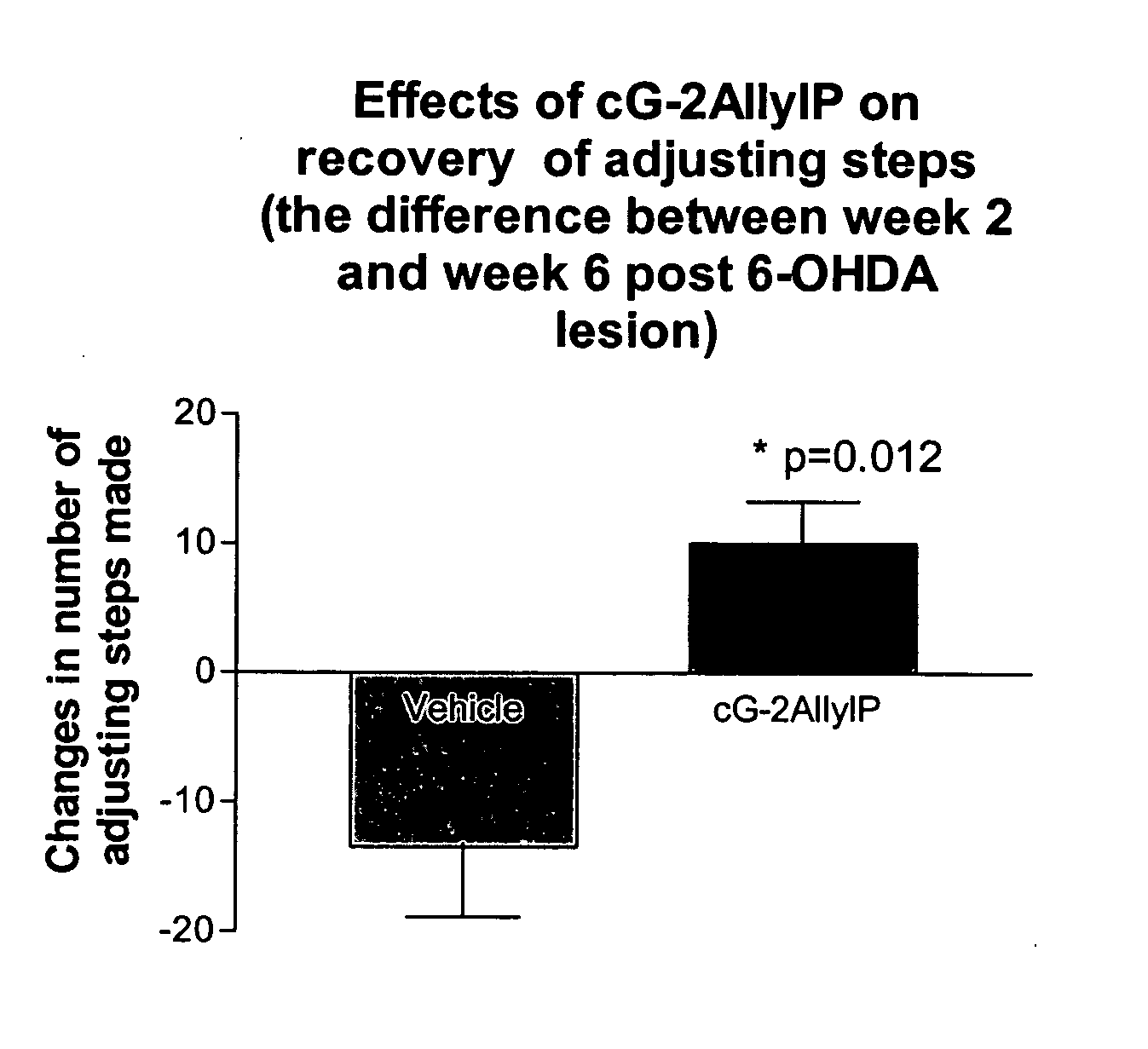
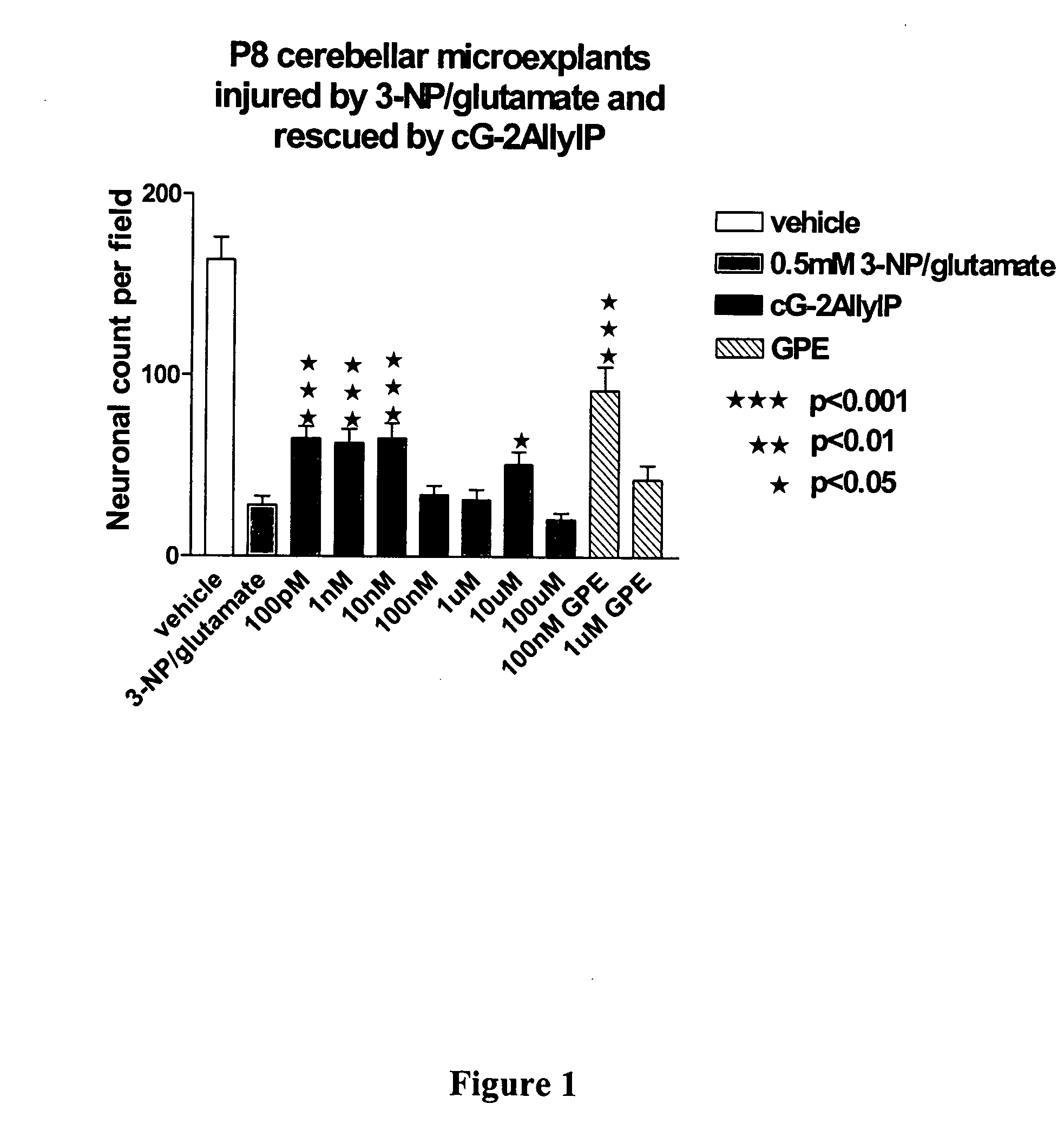
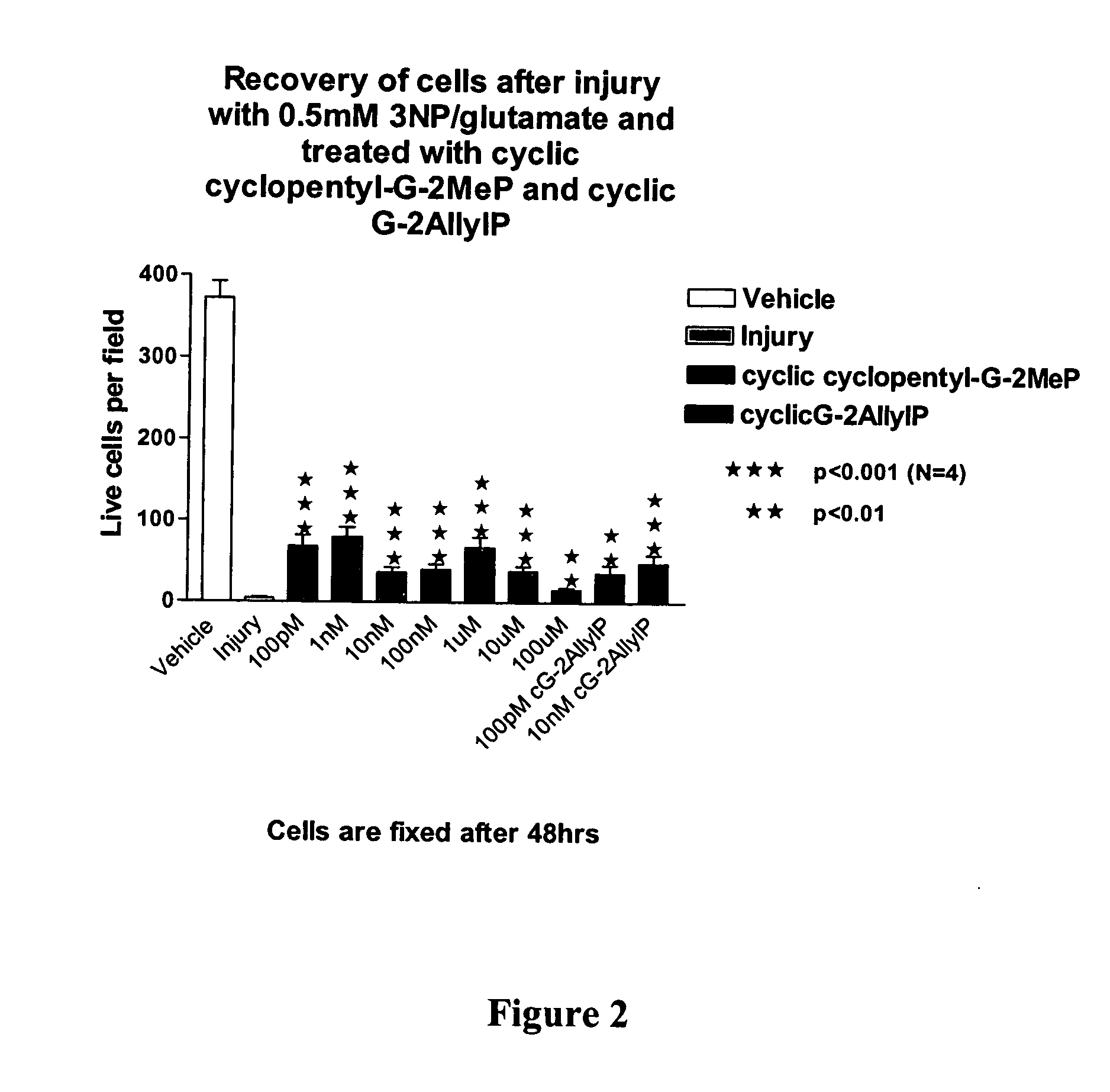
Cyclic G-2AllylProline in treatment of Parkinson's disease
ActiveUS20060258663A1Prevent neuronal injuryReduce tissue damageBiocideNervous disorderNeurophysinsDopamine
Embodiments of this invention provide methods for thereapeutic use of cyclic G-2-Allyl Proline to treat disorders of dopaminergic neurons, including Parkinson's disease. Cyclic G-2Allyl P is neuroprotective and has utility as a therapeutic agent for treatment of diseases and other conditions characterised by degeneration and / or death of dopaminergic neurons and the adverse symptoms of such degeneration and / or death. Such symptoms include loss of cognition and motor function. Compounds are also useful for manufacture of medicaments including tablets, capsules and injectable solutions that are useful for treatment of such conditions.
Owner:NEUREN PHARMA LTD
Method of inducing differentiation of neural stem cell into dopaminergic neuron by using recombinant slow virus
ActiveCN103865956AMultiple differentiation potentialHigh expressionNervous system cellsFermentationSingle cell suspensionApoptosis
The invention relates to bioengineering technologies, and particularly relates a method of inducing differentiation of neural stem cells into dopaminergic neurons by using recombinant slow virus. The method comprises the following steps: constructing a recombinant slow virus vector; separating, culturing and identifying the neural stem cells; transfecting the recombinant slow virus into NSCs; identifying the NSCs transfected by the recombinant slow virus, wherein the process of transfecting the recombinant slow virus into the NSCs comprises the following steps: digesting NSCs after being subjected to three times of passages to prepare a single-cell suspension, and inoculating into a culture plate; transfecting the TH+Brn4 gene recombinant slow virus; adding the slow virus and ploybrene, uniformly shaking, putting the culture plate into an incubator to culture for 1-2 hours, and replacing fresh differential culture medium; meanwhile, screening by using puromycin to obtain stable transfected cells. The method has the advantages as follows: by constructing the slow virus vectors of target genes TH and Brn4, exogenous genes after transfecting the NSCs can be stably expressed in cells; high proportion of TH positive dopaminergic neuron is generated after differentiation; the Brn4 can promote high expression of neurotrophic factors and has the function of inhibiting apoptosis.
Owner:HELP STEM CELL INNOVATIONS CO LTD
Sporoderm-broken germination-activated ganoderma lucidum spores for protection of dopaminergic neurons and treatment of Parkinson's disease
InactiveUS7357933B2Improves tyrosine hydrolase (TH) activitySpeed up the conversion processBiocideLichen medical ingredientsDiseaseMammal
This invention provides a method for treating Parkinson's disease (PD), particularly early stage of PD by orally administering to a mammal sporoderm-broken germination activated Ganoderma lucidum spore powders (GASP) to reduce and / or release the symptom of rotatory behavior in PD, to reduce the progression of neuron apoptosis and to improve the tyrosine hydroxylase (TH) activity so as to increase the conversion of hydroxydopamine (DOPA) to dopamine in the dopaminergic neurons. This invention also provides a method for reducing neuron apoptosis and a method for improving TH activity in dopaminergic neurons by orally administering to a mammal GASP.
Owner:ENHAN TECH HLDG INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com