Dopaminergic neurons and proliferation-competent precursor cells for treating Parkinson's disease
a technology of dopaminergic neurons and precursor cells, applied in the field of dopaminergic neurons and proliferation-competent precursor cells for treating parkinson's disease, can solve the problems of affecting the normal functioning of the nervous system, the inability to reverse the damage of the disease, and the devastating consequences of the disease for those affected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
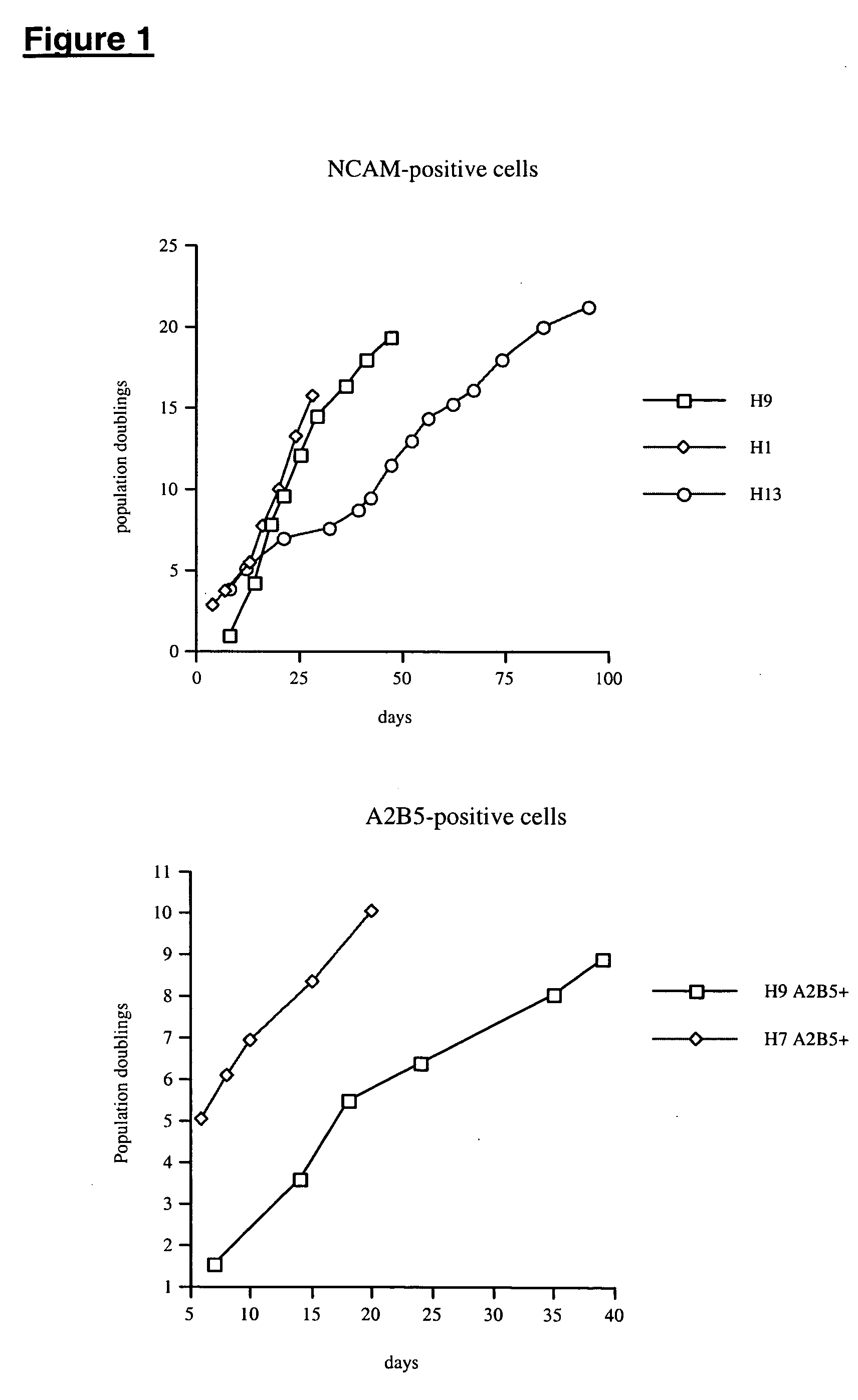
NCAM-Positive Cells
This experiment focused on determining whether the human embryonic stem cells (hES) could undergo directed differentiation to NCAM-positive progenitor cells.
hES cells were harvested either from cultures supported by embryonic fibroblasts, or from feeder-free cultures, as described previously (AU 729377; WO 01 / 51616). Embryoid bodies were produced as follows. Confluent monolayer cultures of hES cells were harvested by incubating in 1 mg / mL collagenase for 5-20 min, following which the cells are scraped from the plate. The cells were then dissociated into clusters and plated in non-adherent cell culture plates (Costar) in a medium composed of 80% KO (“knockout”) DMEM (Gibco) and 20% non-heat-inactivated FBS (Hyclone), supplemented with 1% non-essential amino acids, 1 mM glutamine, 0.1 mM β-mercaptoethanol. The cells are seeded at a 1:1 or 1:2 ratio in 2 mL medium per well (6 well plate).
After 4-8 days in suspension, the EBs were plated intact in DMEM / F12 mediu...
example 2
A2B5-Positive Cells
Cells in this experiment were immunoselected for the surface marker A2B5. hES cells were induced to form EBs in 20% FBS. After 4 days in suspension, the EBs were plated onto fibronectin in DMEM / F12 with N2 and B27 supplemented with 10 ng / mL human EGF, 10 ng / mL human bFGF, 1 ng / mL human IGF-I, and 1 ng / mL human PDGF-AA. After 2-3 days in these conditions, 25-66% of the cells express A2B5. This population is enriched by magnetic bead sorting to 48-93% purity (Table 2).
TABLE 2Differentiation and Sorting Conditions for A2B5-positive CellsCells staininghESFactors used inpositively for NCAMCell Line used forDifferentiationBeforePositiveNegativeDifferentiationCultureType of SortsortsortsortH7 p32 667.004C F N I Pbead sort257710H1 p43 667.010C F N I Pbead sort62n / a50H1 p44 667.012C F N I Pbead sort568932H1 p46 667.020E P F Ibead sort2748 2H1 p47 667.032E P F Ibead sort579330H9 p40MG 667.038E P F Ibead sort669341H9 p42 667.041E P F Ibead sort2770 6
Factor abbreviations:...
example 3
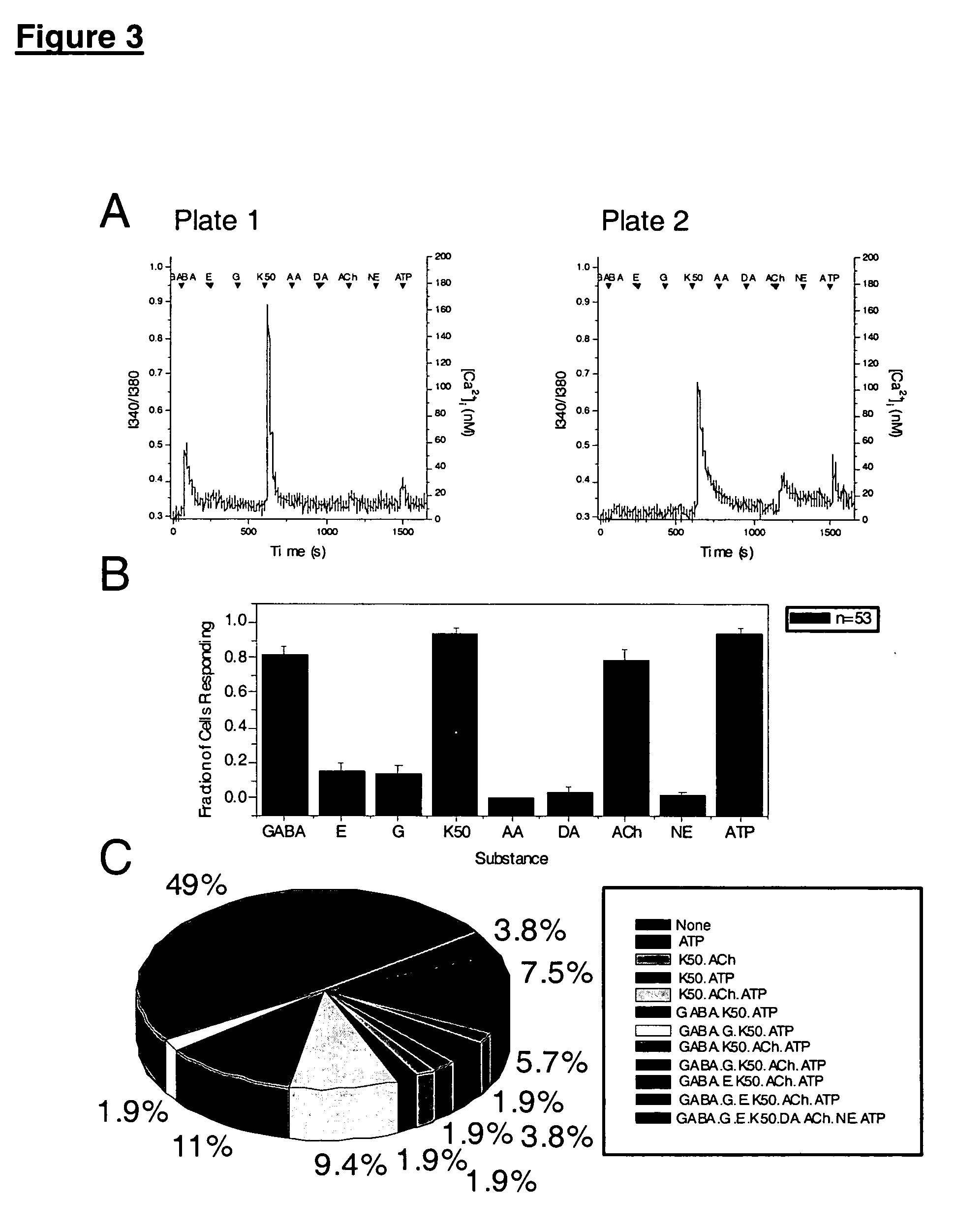
Differentiation to Mature Neurons
To generate terminally differentiated neurons, the first stage of differentiation was induced by forming embryoid bodies in FBS medium with or without 10 μM retinoic acid (RA). After 4 days in suspension, embryoid bodies were plated onto fibronectin-coated plates in defined medium supplemented with 10 ng / mL human EGF, 10 ng / mL human bFGF, 1 ng / mL human PDGF-AA, and 1 ng / mL human IGF-1. The embryoid bodies adhered to the plates, and cells began to migrate onto the plastic, forming a monolayer.
After 3 days, many cells with neuronal morphology were observed. The neural precursors were identified as cells positive for BrdU incorporation, nestin staining, and the absence of lineage specific differentiation markers. Putative neuronal and glial progenitor cells were identified as positive for polysialylated NCAM and A2B5. Forty one to sixty percent of the cells expressed NCAM, and 20-66% expressed A2B5, as measured by flow cytometry. A subpopulation of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com