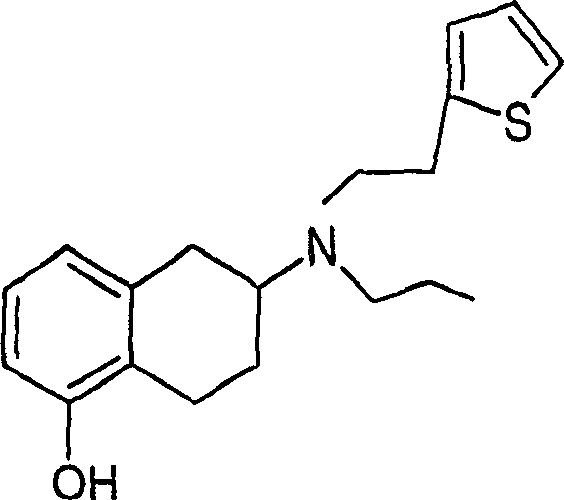
Use of rotigotine for treatment or prevention of dopaminergic neurone loss
A dopaminergic and useful technology, applied in the field of use of rotigotine for treating or preventing the loss of dopaminergic neurons, can solve the problem that the preventive treatment of Parkinson's disease cannot be guaranteed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Embodiment 1: Rotigotine plaster
[0121] 1.8 g rotigotine (free base) was dissolved in 2.4 g ethanol and 0.4 g Kollidon 90F (dissolved in 1 g ethanol) was added. This mixture was added to a 74% solution of silicone polymer in heptane (8.9 g BioPSA 7-4201 + 8.9 g BIO-PSA 7-4301 [DowComing]). After adding 2.65 g of petroleum ether, the mixture was stirred at 700 rpm for 1 hour in order to obtain a homogeneous dispersion. After layering in polyester, it was dried at 50°C. The final weight of the plaster was 50 g / cm2.
Embodiment 2
[0122] Example 2. Rotigotine depot suspension
[0123] (a) Weigh 1411.2 g of Miglyol 812 into a Duran flask. 14.4 g of Imwitor 312 were added to the Miglyol and heated to 80°C for 30 minutes with stirring. The clear solution was cooled to room temperature and filtered.
[0124] (b) 1188 g of the solution produced in (a) were transferred to a glass laboratory reactor, 12 g of rotigotine were added and homogenized in an Ultraturrax at 10,000 rpm under nitrogen for 10 minutes. The suspension was poured gently into a brown glass bottle while running the Ultraturrax (2,000 rpm).
Embodiment 3
[0125] Example 3: Subacute MPTP Model
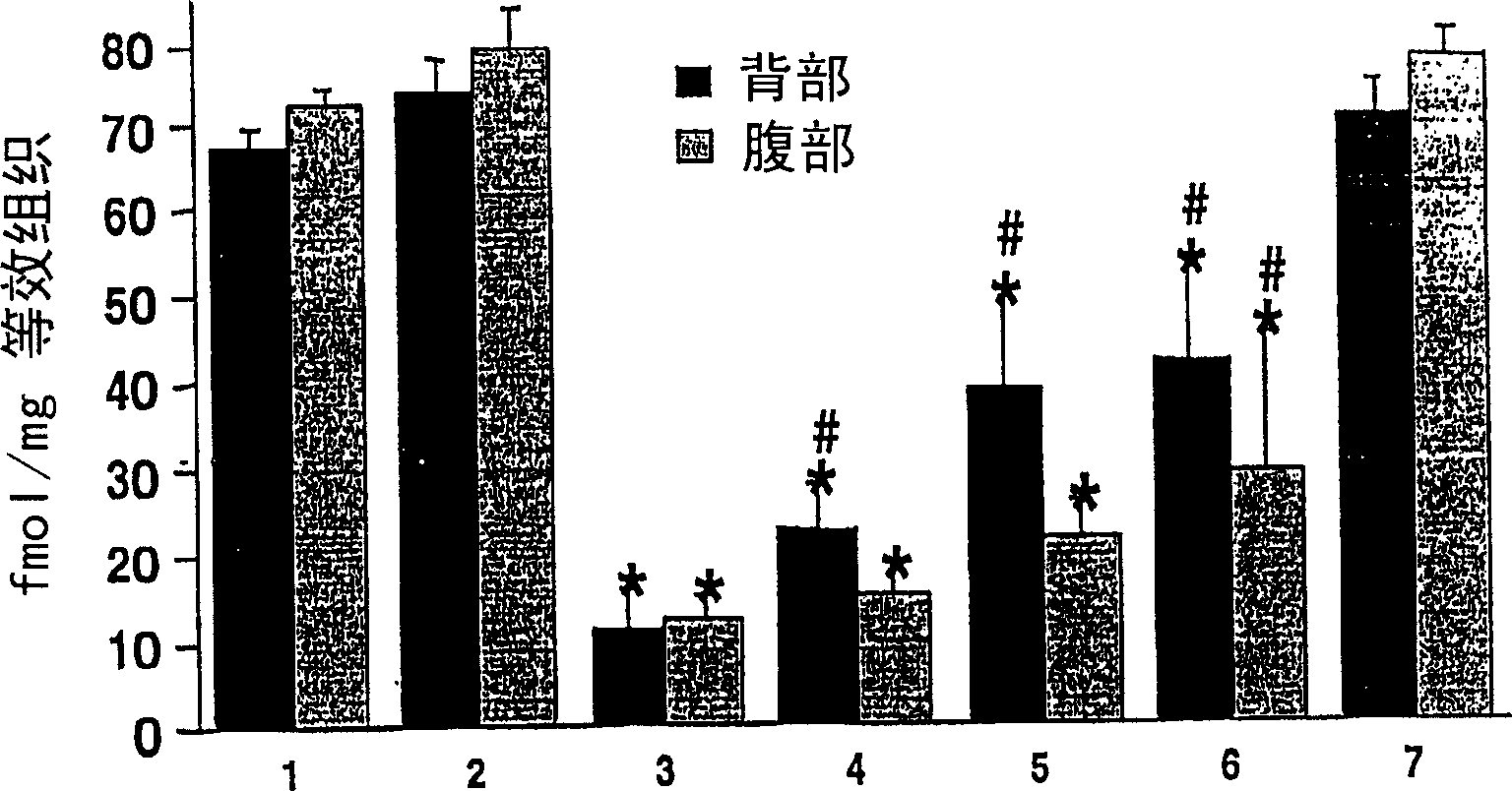
[0126] For intoxication purposes, 80 mg / kg of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP) was administered to mice (20 mg / kg in batches at two intervals). hours, groups 3 to 6 in Figures 1 and 2), which can lead to about 50 to 60% of neuron degeneration in the substantia nigra (group 3 in Figures 1 and 2 has the greatest degeneration). Rotigotine was administered daily for 7 days at doses of 0.3, 1 or 3 mg / kg as a so-called "sustained release formulation" (see Example 2) (groups 4 to 6 in Figures 1 and 2). A rotigotine vehicle solution (see Example 2 without rotigotine HCl) was administered to a group of MPTP-treated animals (group 3) and served as reference. Groups 1, 2 and 7 served as controls whereby group 1 received no treatment at all, group 2 was treated with a vehicle solution of MPTP and rotigotine and group 7 received rotigotine alone. Animals were sacrificed on day 8 and their brains were removed and f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com