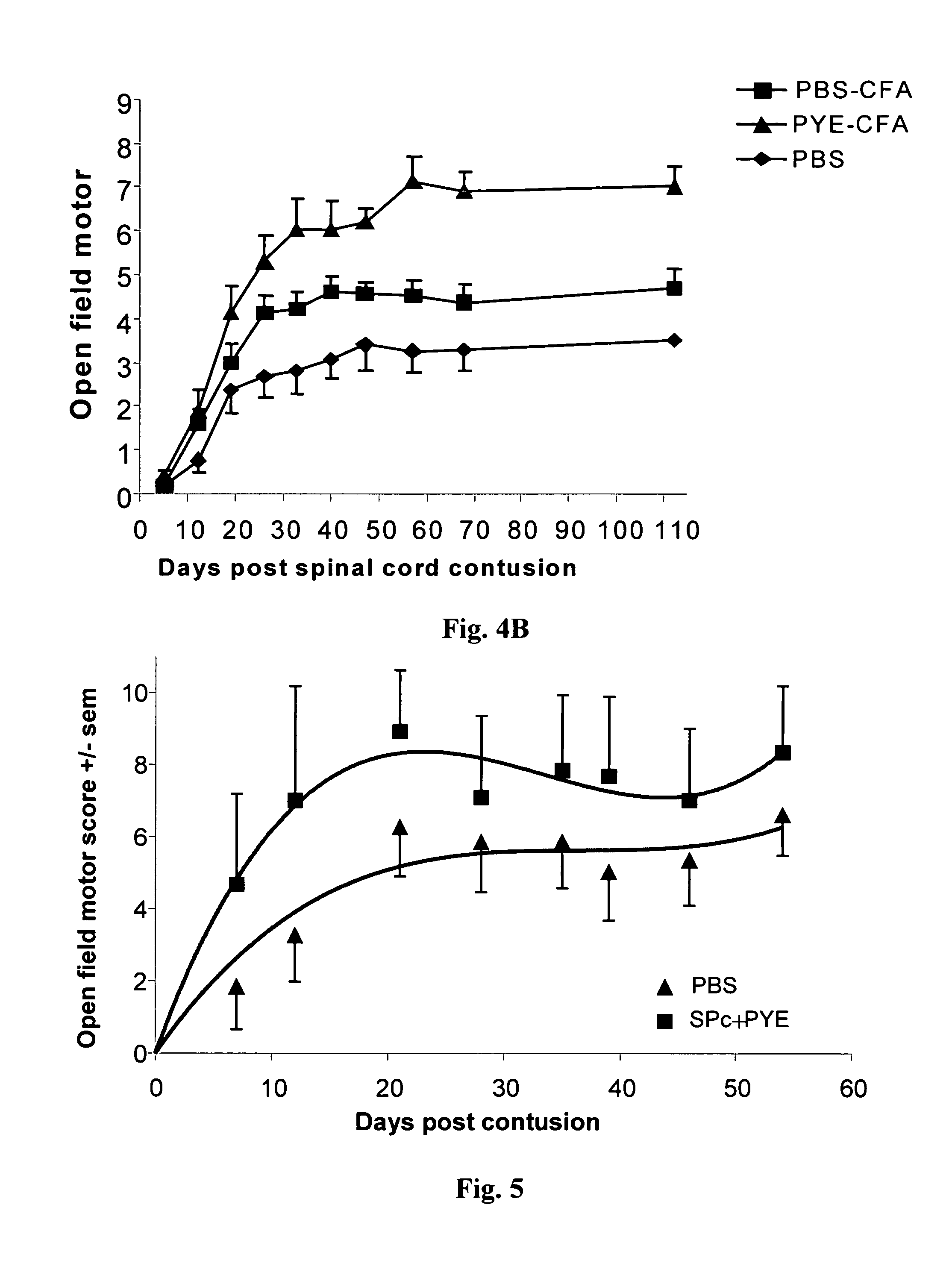
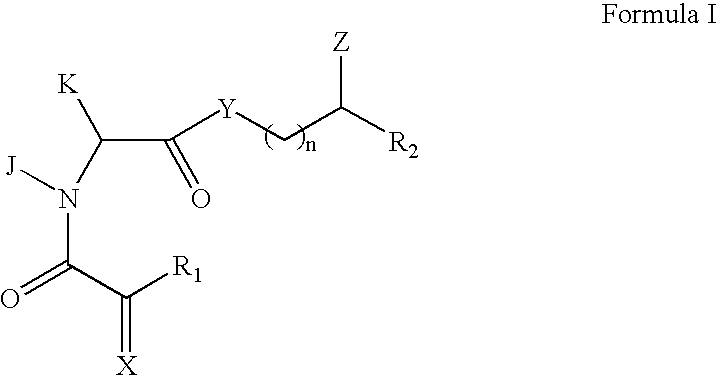
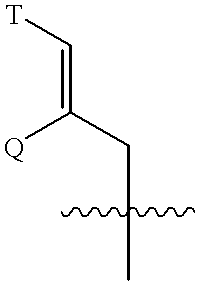
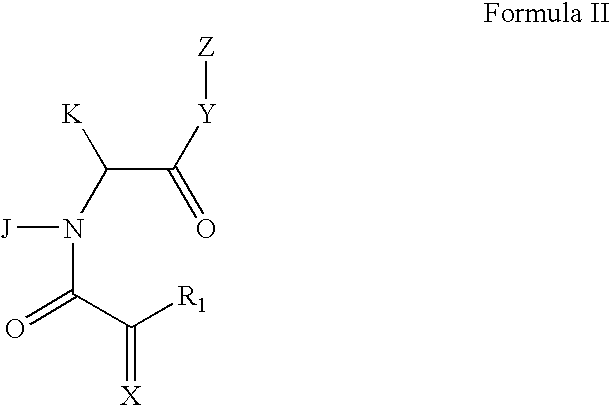
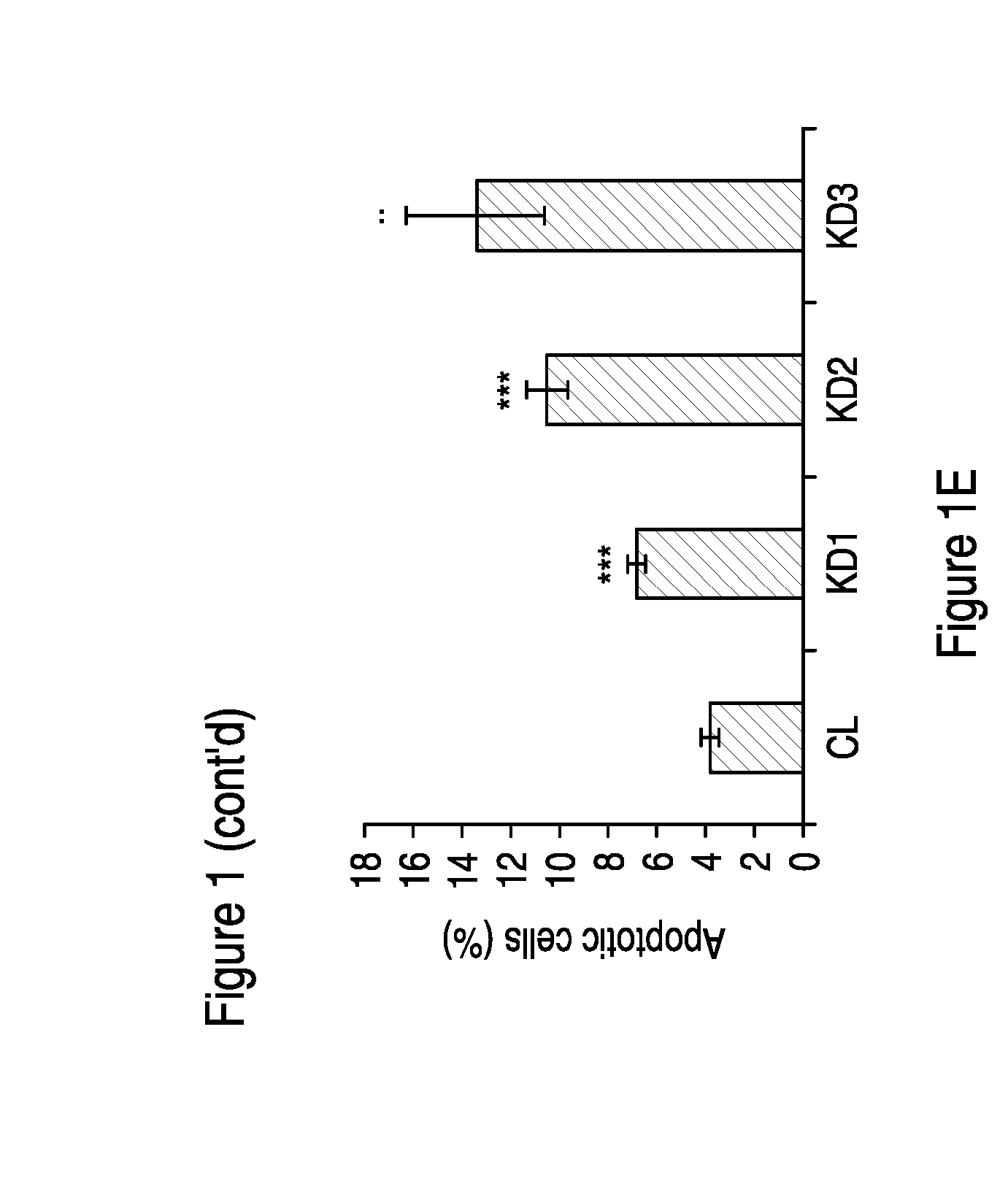
Patents
Literature
92 results about "Neuronal degeneration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neuronal Degeneration. An inherited neurological disease, called “Neuronal Degeneration” or NDG, has been reported in Great Pyrenees dogs. The age-of-onset of this disease is very young, well before an affected dog’s first birthday, but begins quite mildly. Initial signs include slipping, sliding, and difficulty maneuvering on smooth surfaces.
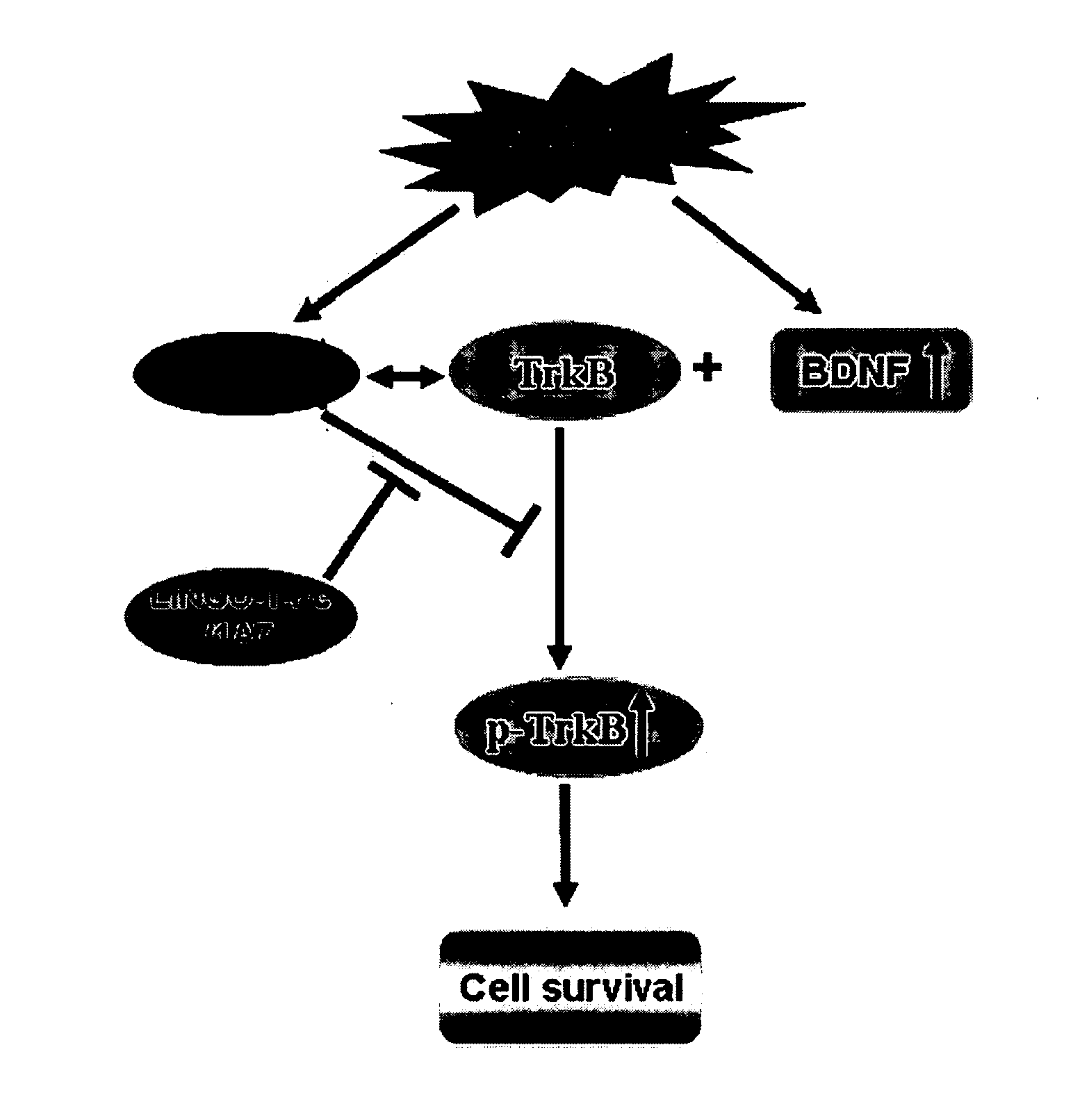
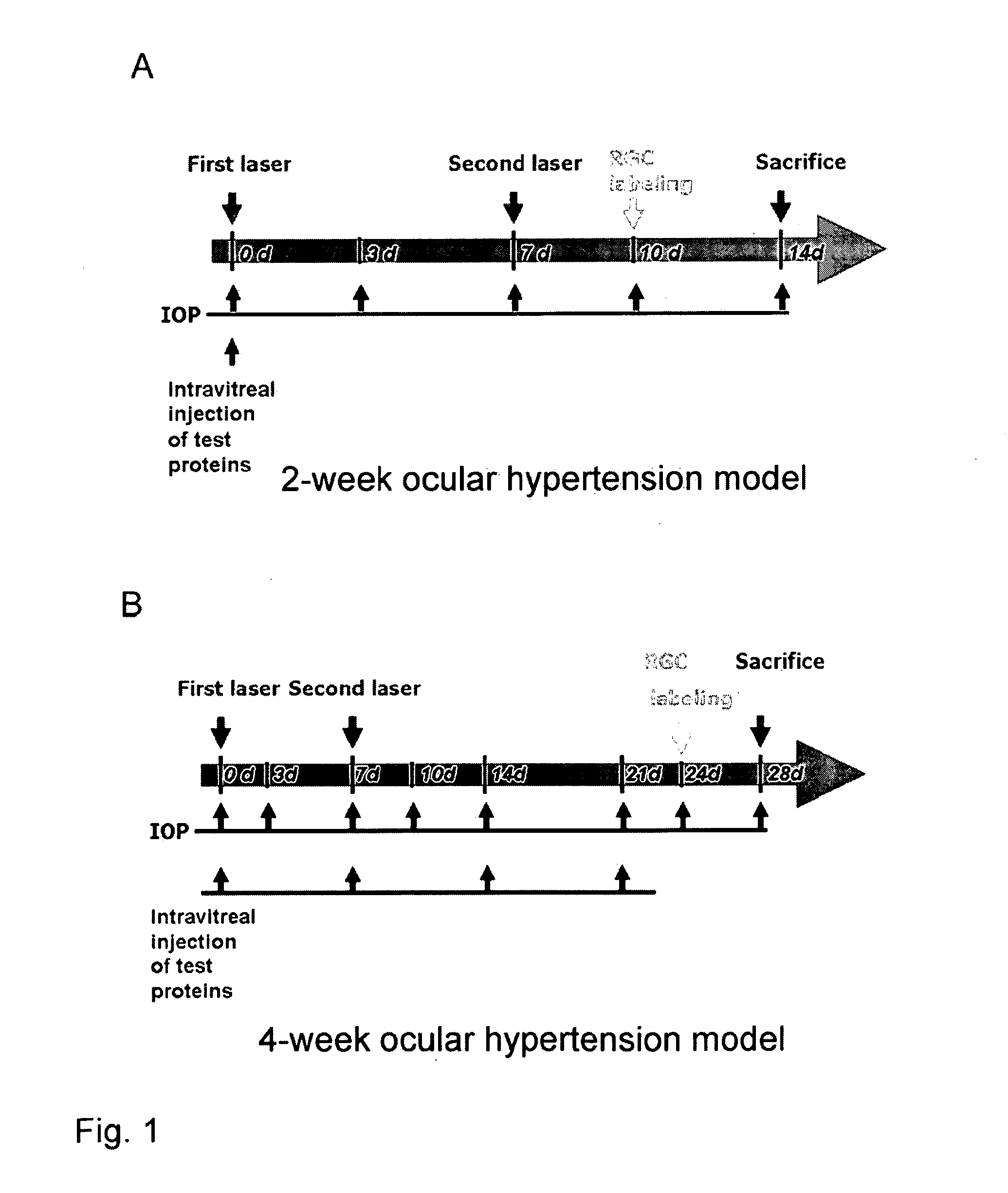
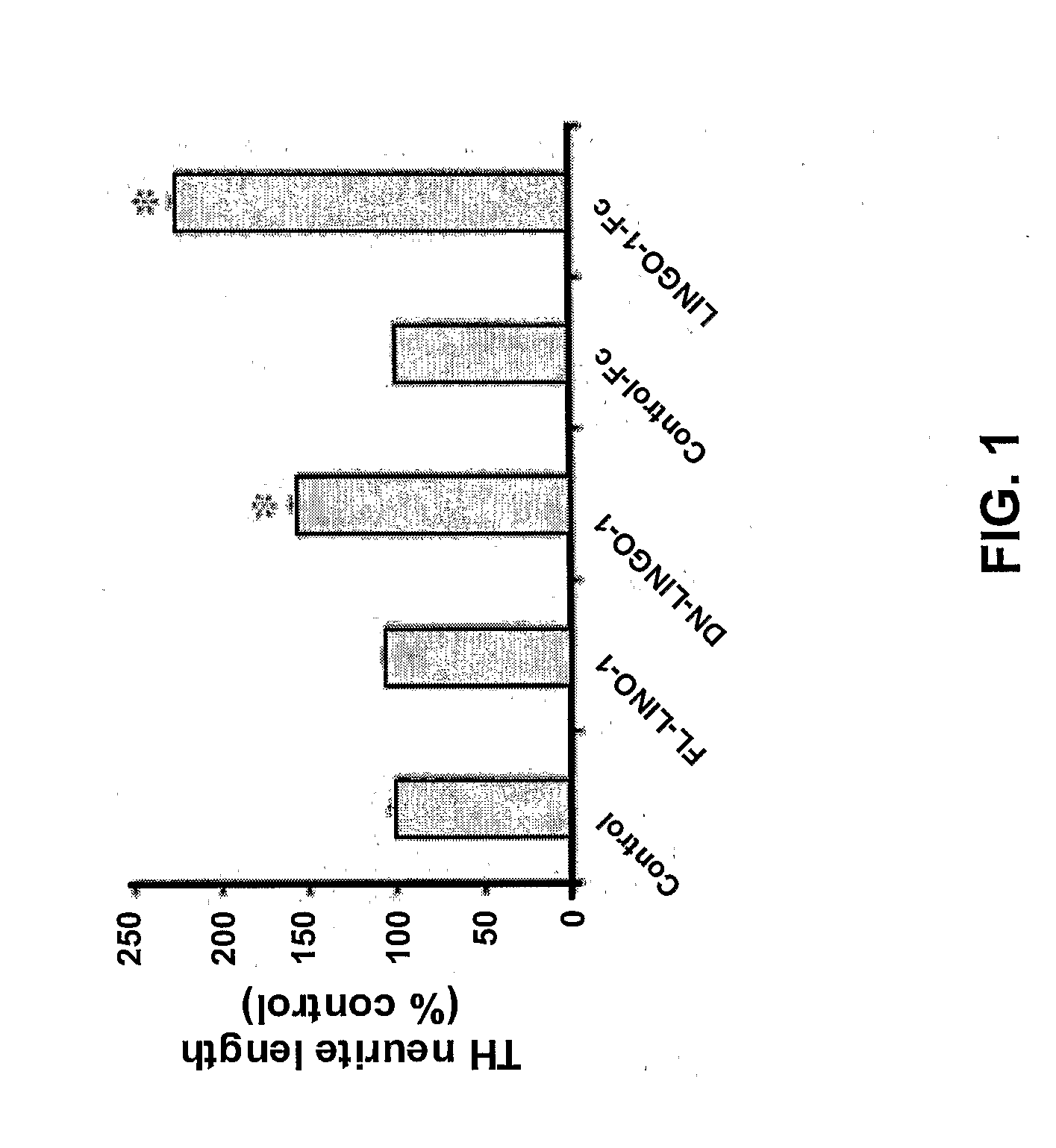
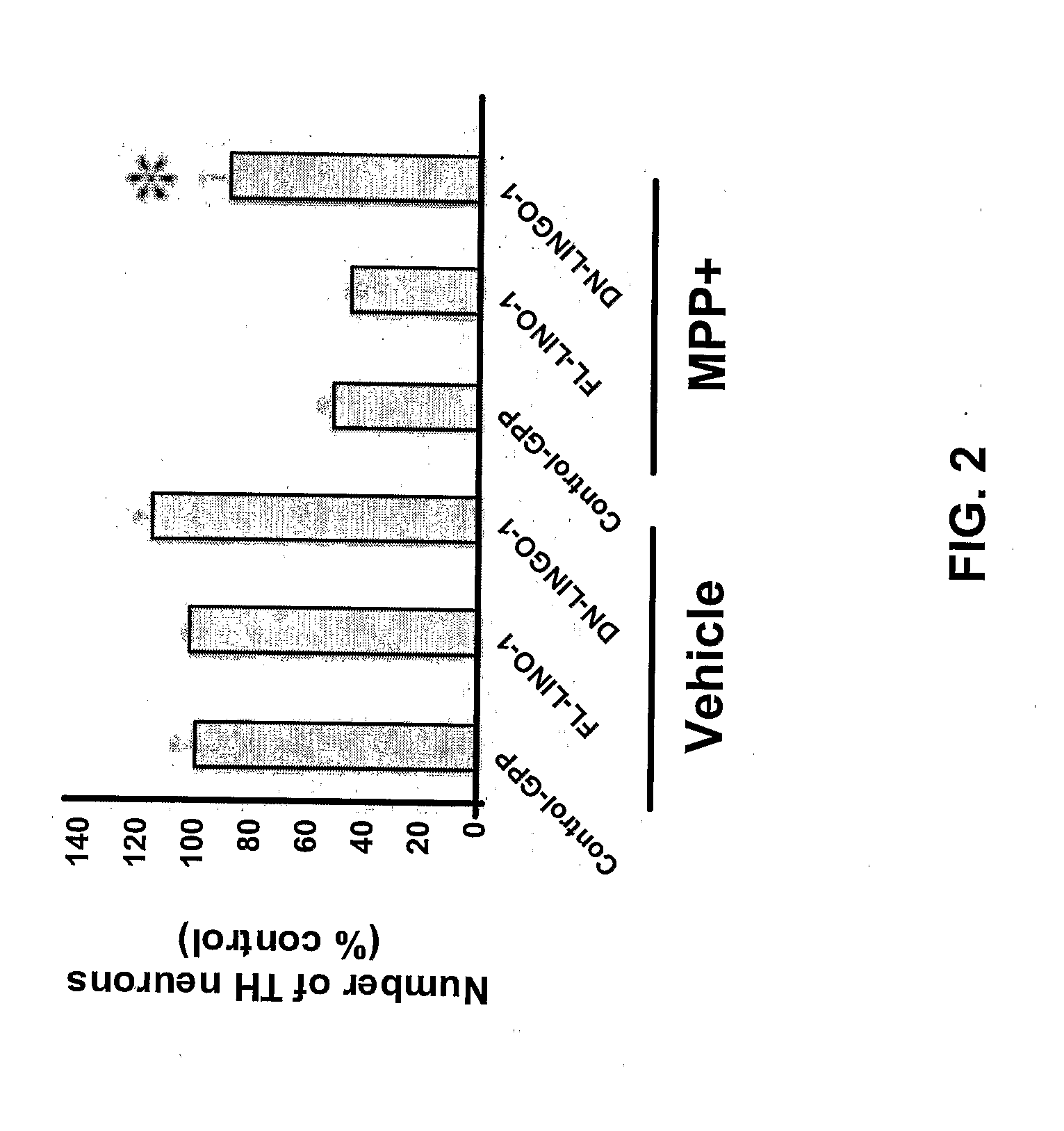
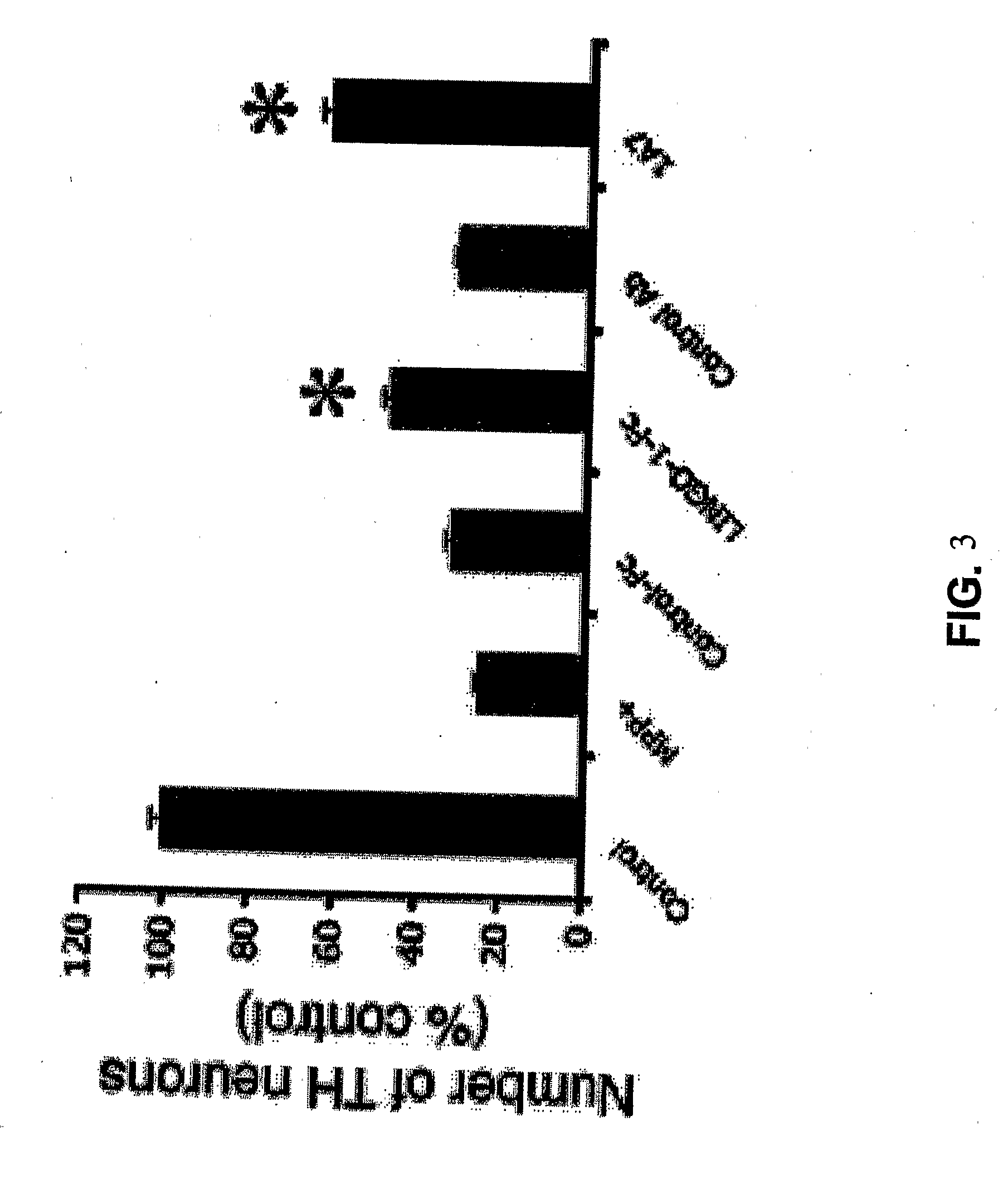
Methods for Treating Pressure Induced Optic Neuropathy, Preventing Neuronal Degeneration and Promoting Neuronal Cell Survival Via Administration of LINGO-1 Antagonists and TrkB Agonists
InactiveUS20100297121A1High activityPromote cell activationOrganic active ingredientsSenses disorderNeuronal degenerationMedicine
This invention relates to methods for promoting neuronal survival and regeneration using LINGO-1 antagonists and TrkB agonists. Additionally, the invention relates to methods for treating pressure induced optic neuropathies using LINGO-1 antagonists. The invention also relates generally to methods for increasing TrkB activity and inhibiting JNK pathway signaling using a LINGO-1 antagonist.
Owner:BIOGEN MA INC
Methods for Promoting Neurite Outgrowth and Survival of Dopaminergic Neurons
InactiveUS20090246189A1Promote regenerationPromoting outgrowthOrganic active ingredientsBiocideNeuronal degenerationDopamine
The present invention relates generally to methods for promoting regeneration, outgrowth and survival of dopaminergic neurons comprising contacting said dopaminergic neurons with an effective amount of a composition comprising an Sp35 antagonist. Additionally, the invention is related generally to methods of treating various diseases, disorders or injuries associated with dopaminergic neuronal degeneration or death by administration of an Sp35 antagonist.
Owner:THE MCLEAN HOSPITAL CORP +1
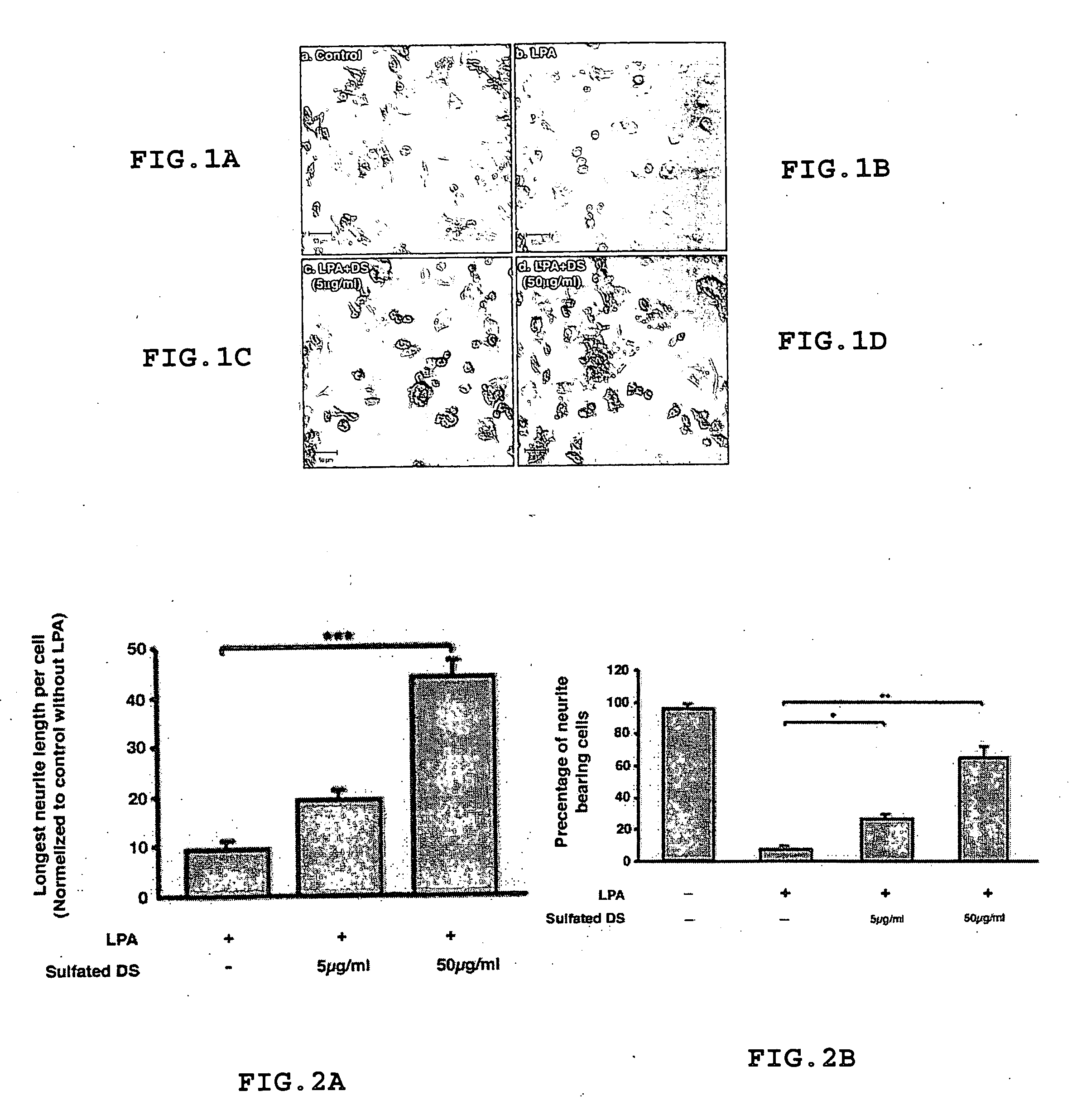
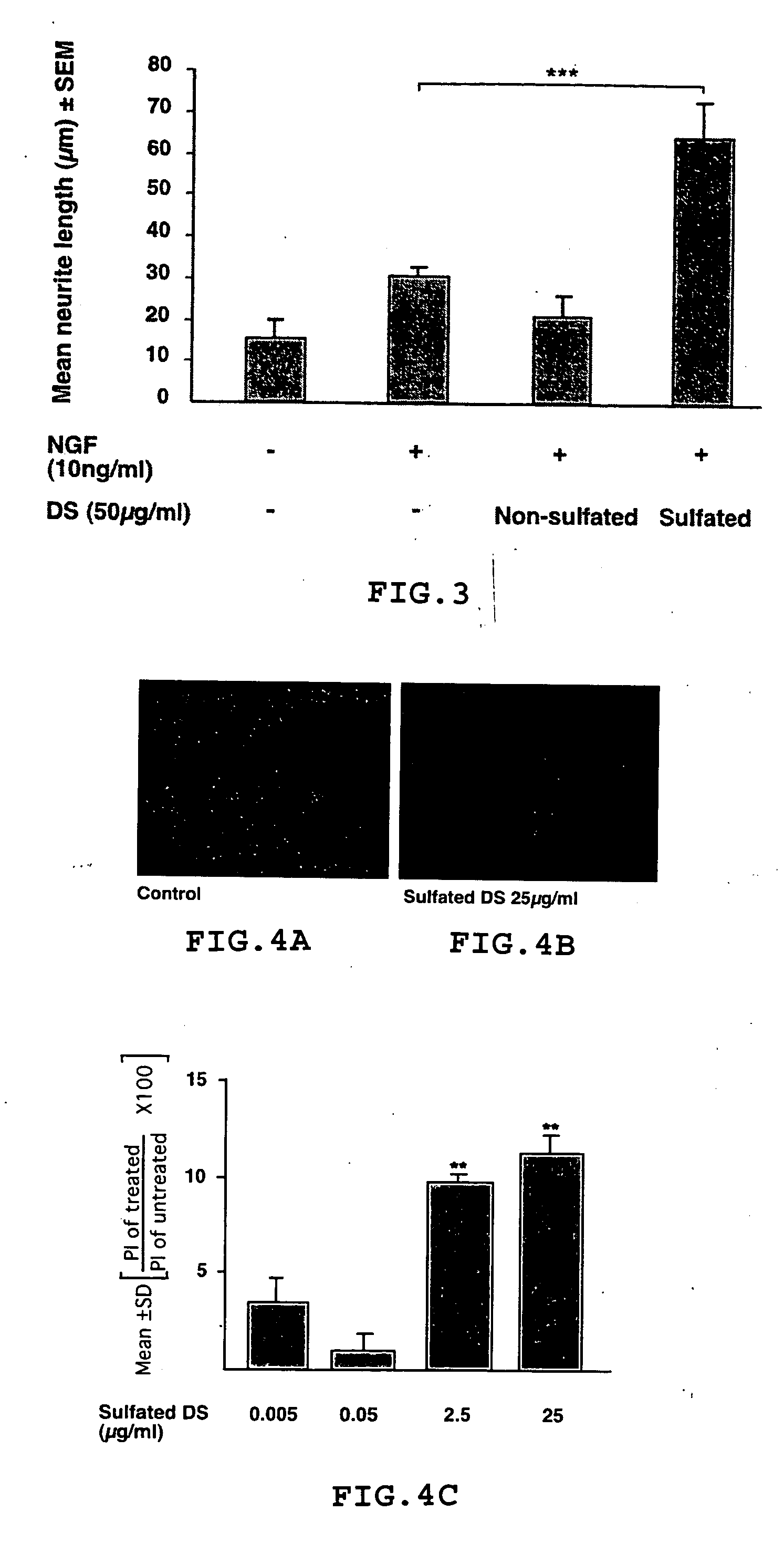
Method for treating or inhibiting the effects of injuries or diseases that result in neuronal degeneration and method for promoting neurogenesis
InactiveUS20070010484A1Promoting neurogenesisTreating and inhibiting and ameliorating effectBiocideOrganic active ingredientsNeuronal degenerationMedicine
Oligosaccharides, and in particular disaccharides, which are degradation products of chondroitin sulfate proteoglycan are effective for use in treating, inhibiting, or ameliorating the effects of injuries or diseases or disorders that result in or are caused by neuronal degeneration or of disorders resulting in mental and cognitive dysfunction. They are also useful for promoting neurogenesis.
Owner:YEDA RES & DEV CO LTD
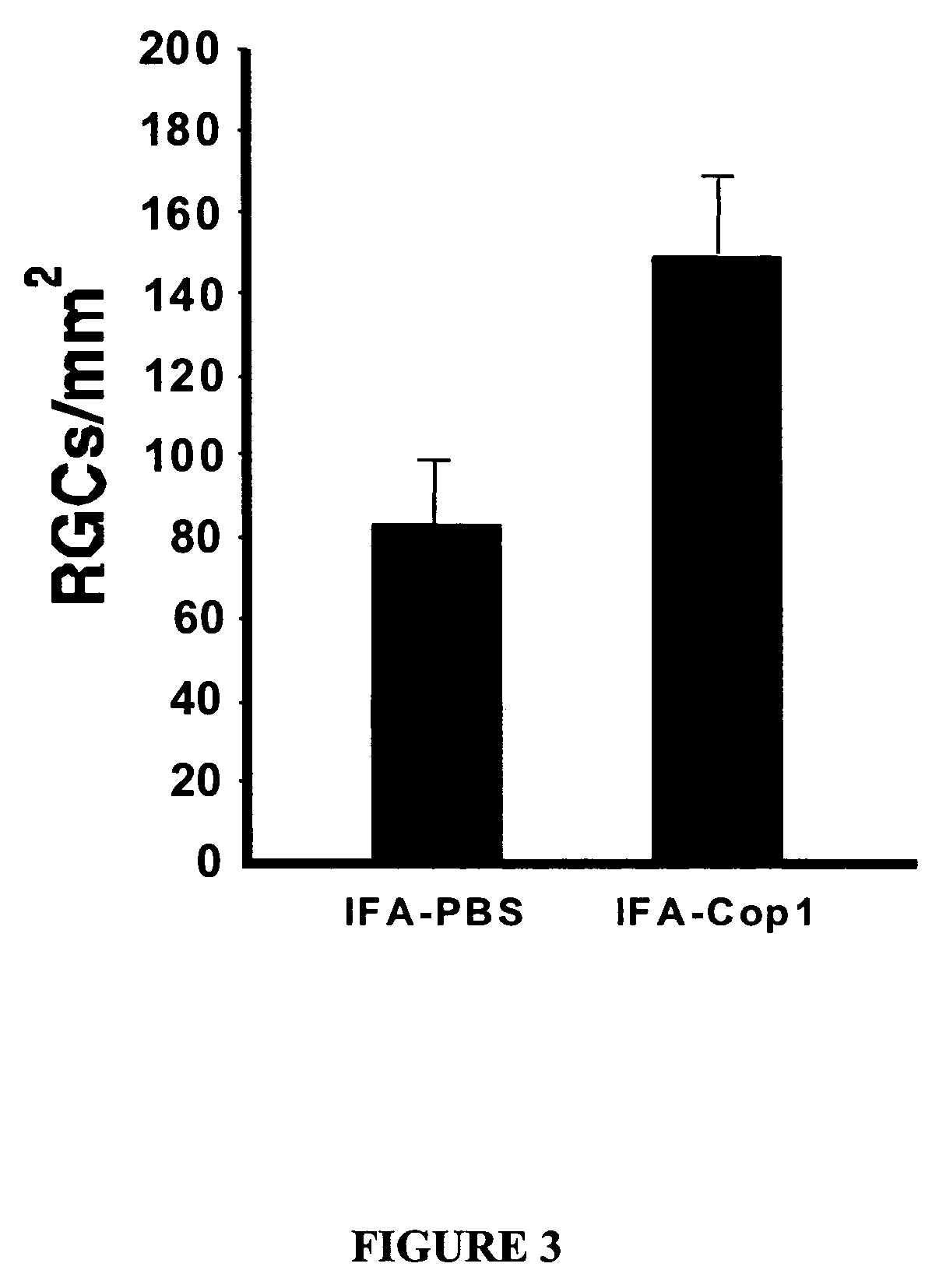
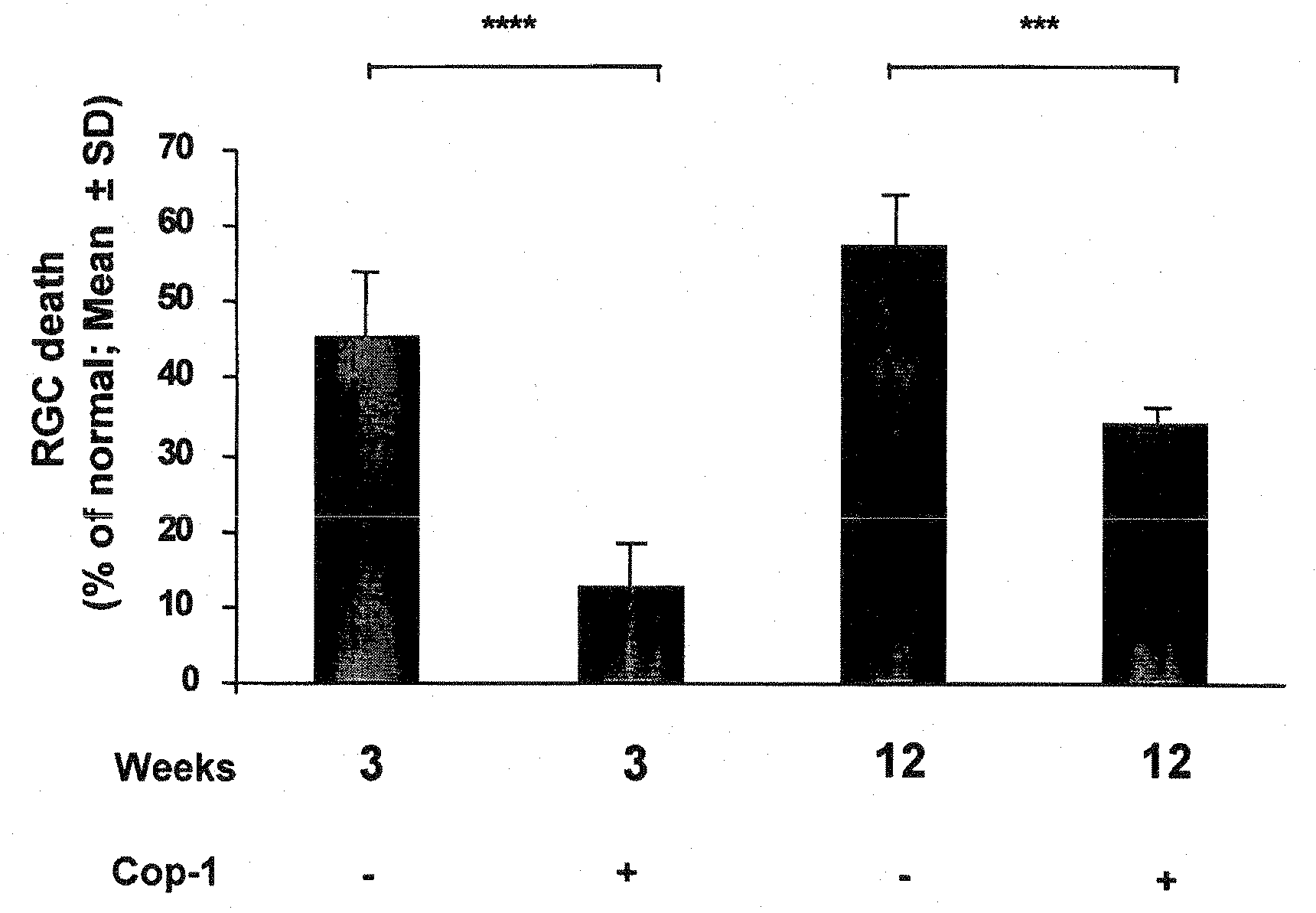
Eye-Drop Vaccine Containing Copolymer 1 for Therapeutic Immunization
InactiveUS20070248569A1Promote nerve regenerationOrganic active ingredientsSenses disorderNervous systemNeuronal degeneration
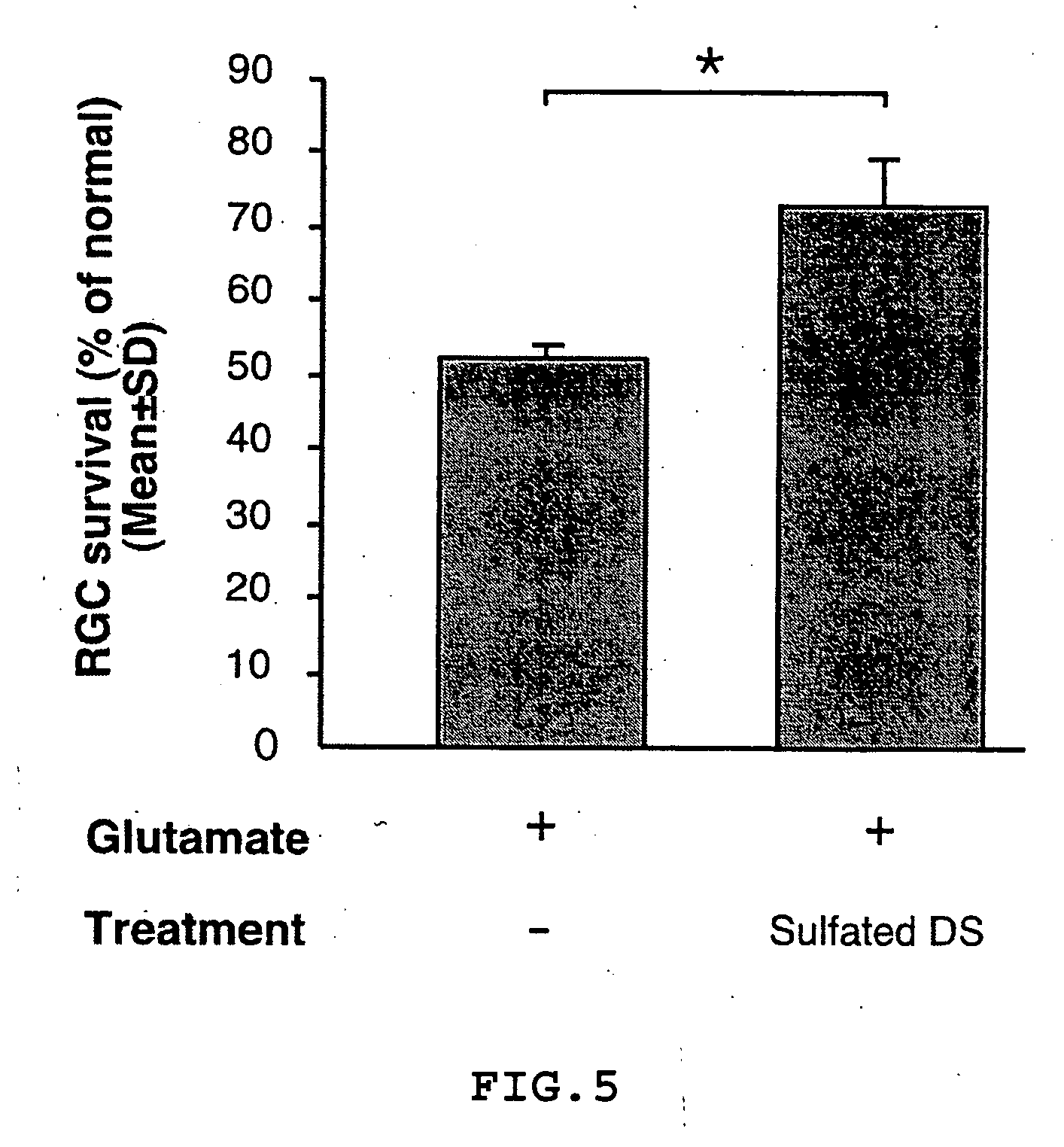
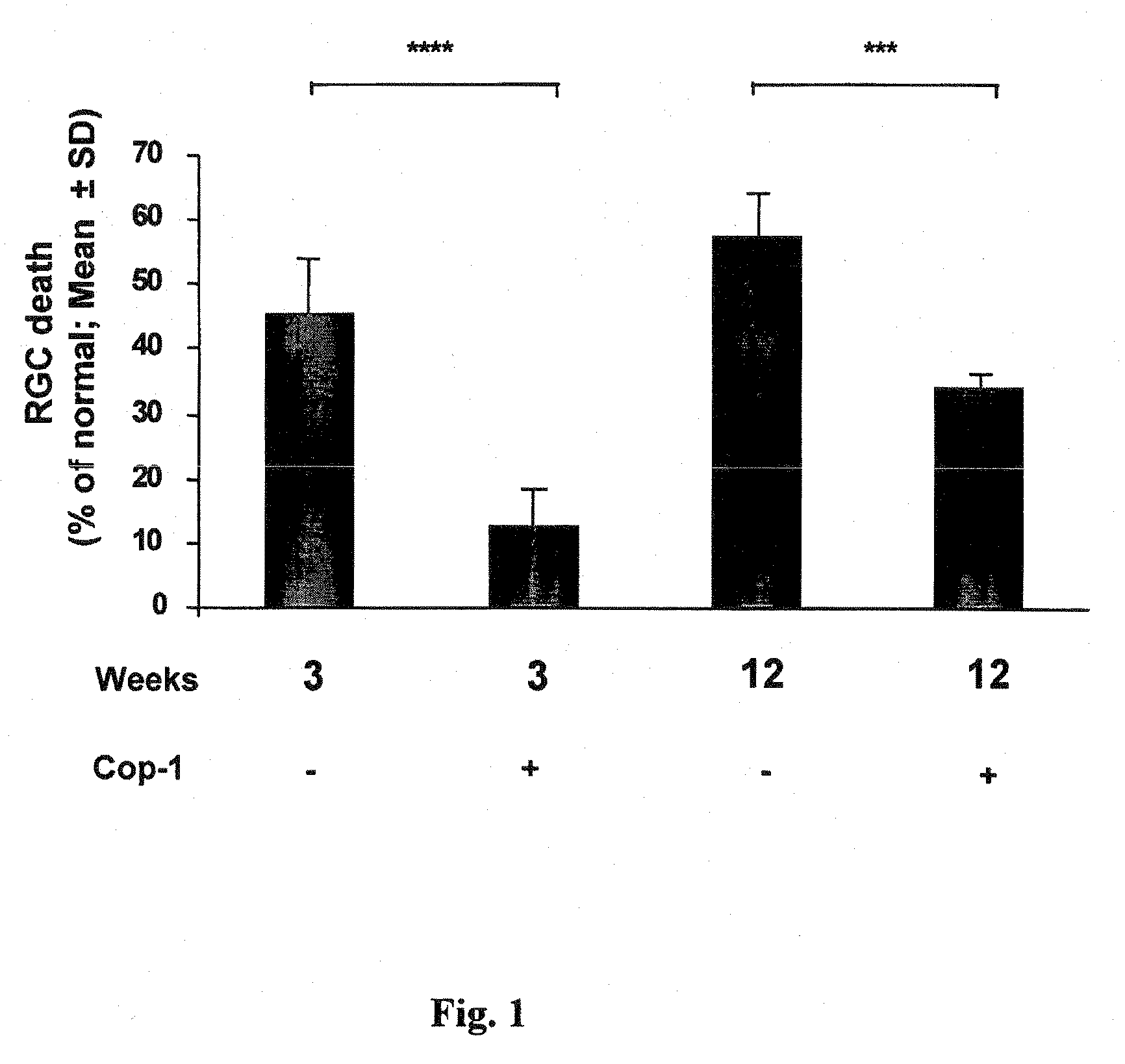
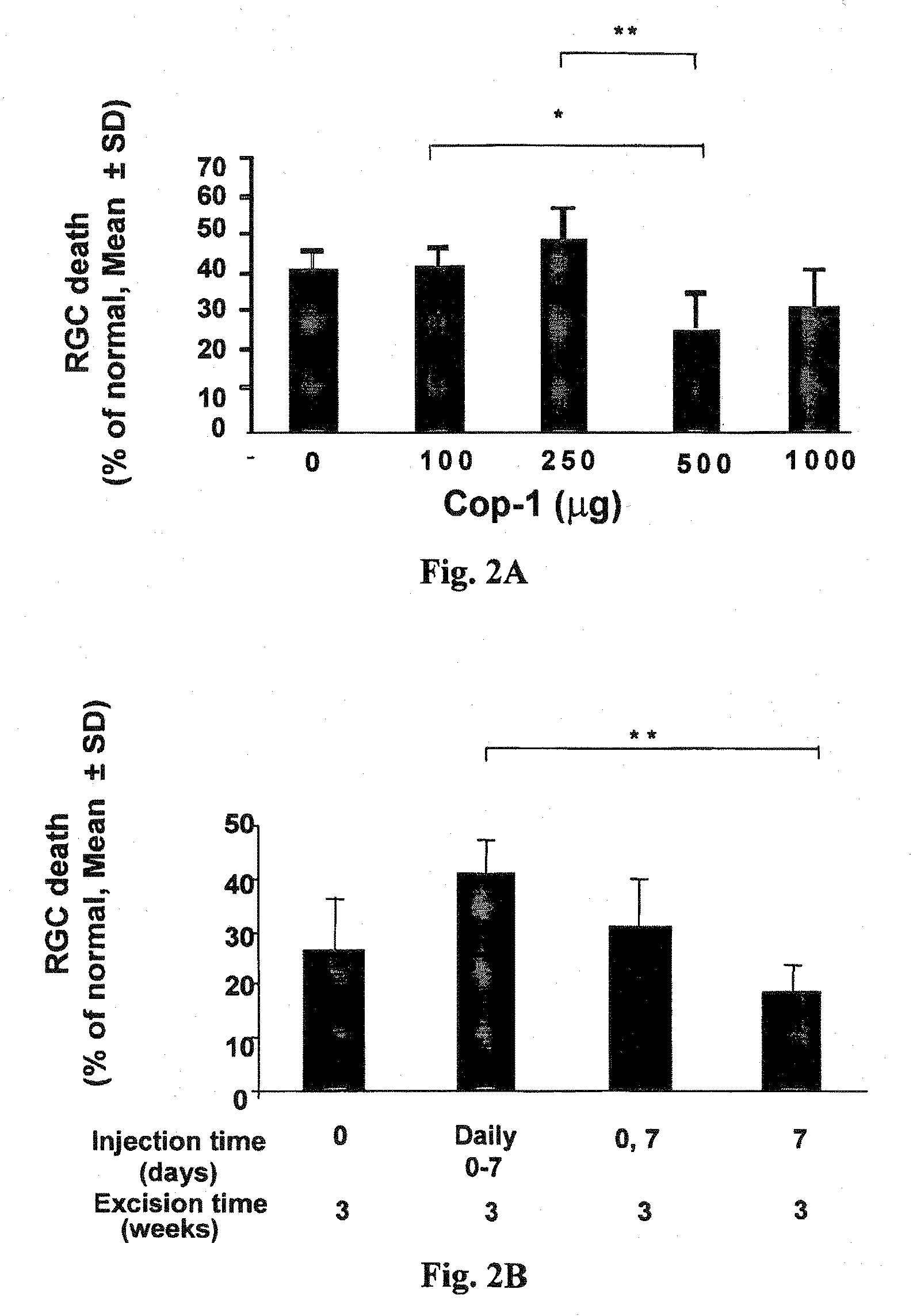
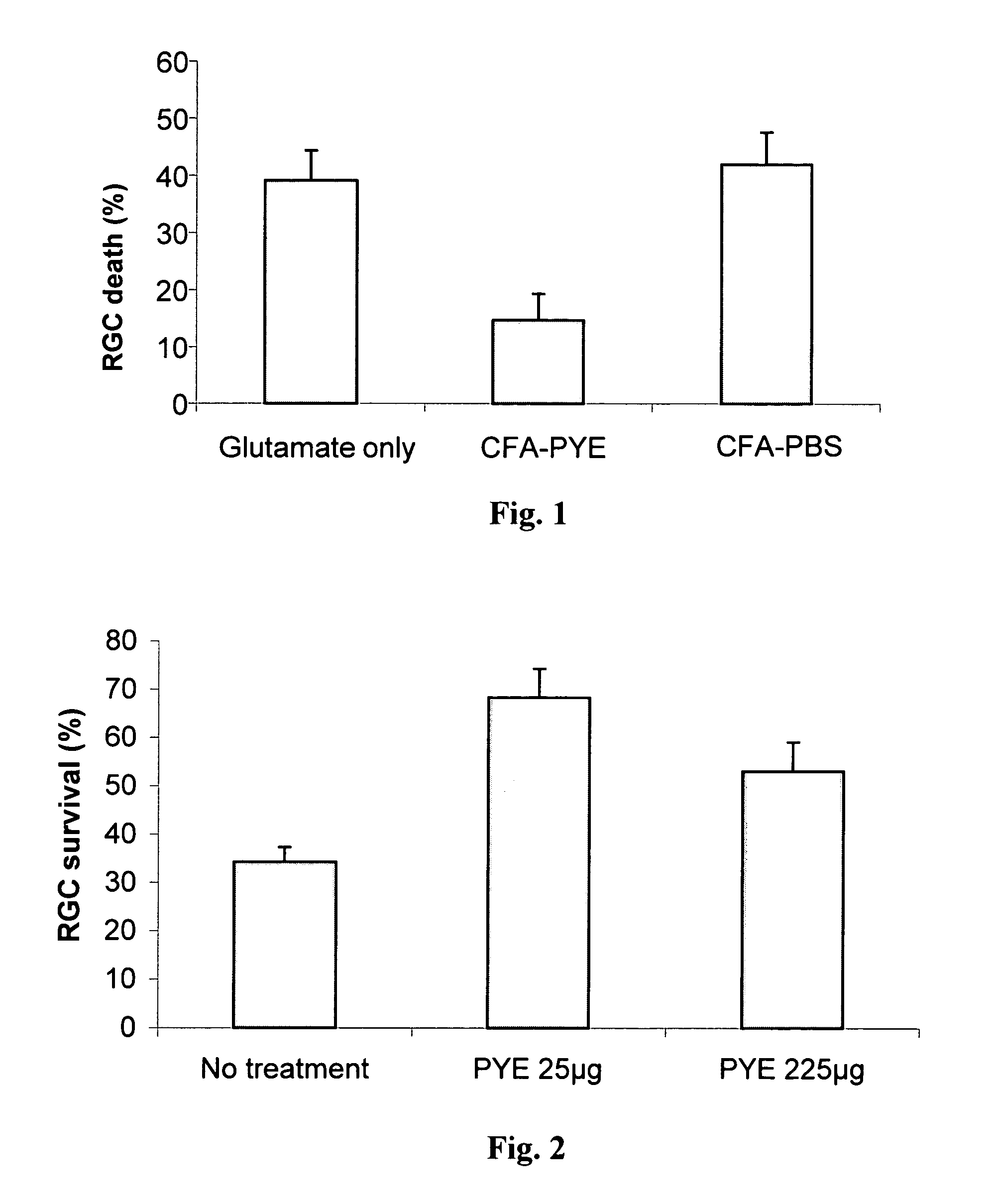
The invention provides an eye-drop vaccine for therapeutic immunization of a mammal comprising Copolymer 1, a Copolymer 1-related peptide, or a Copolymer 1-related polypeptide, for treating neuronal degeneration caused by an injury or disease, disorder or condition in the central nervous system (CNS) or peripheral nervous system (PNS), for preventing or inhibiting neuronal secondary degeneration which may otherwise follow primary injury in the CNS, for promoting nerve regeneration in the CNS or in the PNS after an injury, disease, disorder or condition or for protecting CNS and PNS cells from glutamate toxicity.
Owner:YEDA RES & DEV CO LTD
Method for detecting neuronal degeneration and anionic fluorescein homologue stains therefor
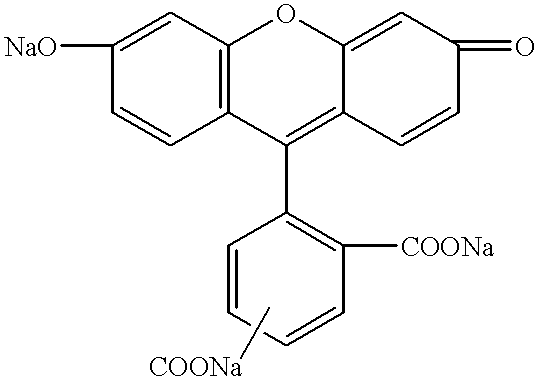
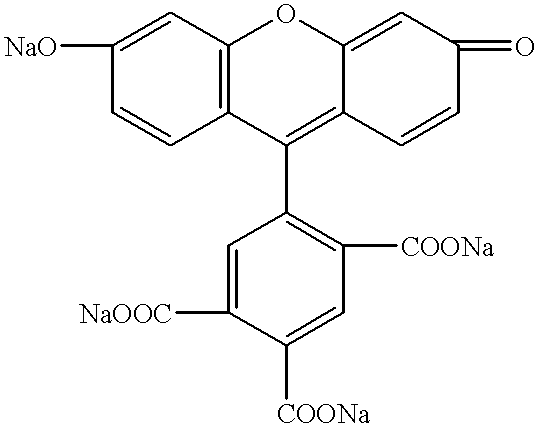
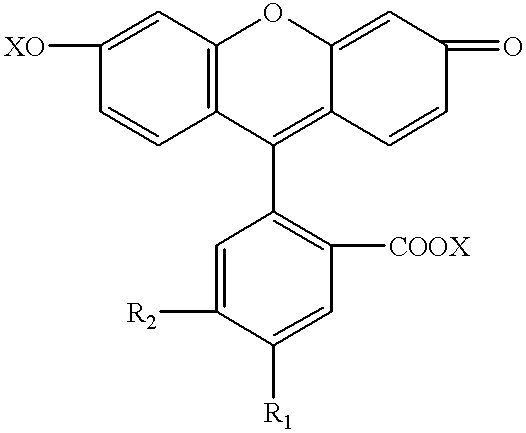
InactiveUS6229024B1Simple and reliable to useSensitive highOrganic chemistryDiaryl/thriaryl methane dyesNeuronal degenerationFluorescein
An anionic fluorescein homologue capable of selectively staining degenerating neurons in brain slices, the method of making the anionic fluorescein homologue, and the method of using the same for detecting neuronal degeneration. Two anionic fluorescein based fluorescent stains are the water soluble tribasic salt of a mixture of the fluorescein isomers 5-carboxyfluorescein and 6-carboxyfluorescein, and the water soluble tetrabasic salt of 5,6 dicarboxyfluorescein.
Owner:SCHMUED LAURENCE C
Methods and Compositions to Inhibit P2x7 Receptor Expression
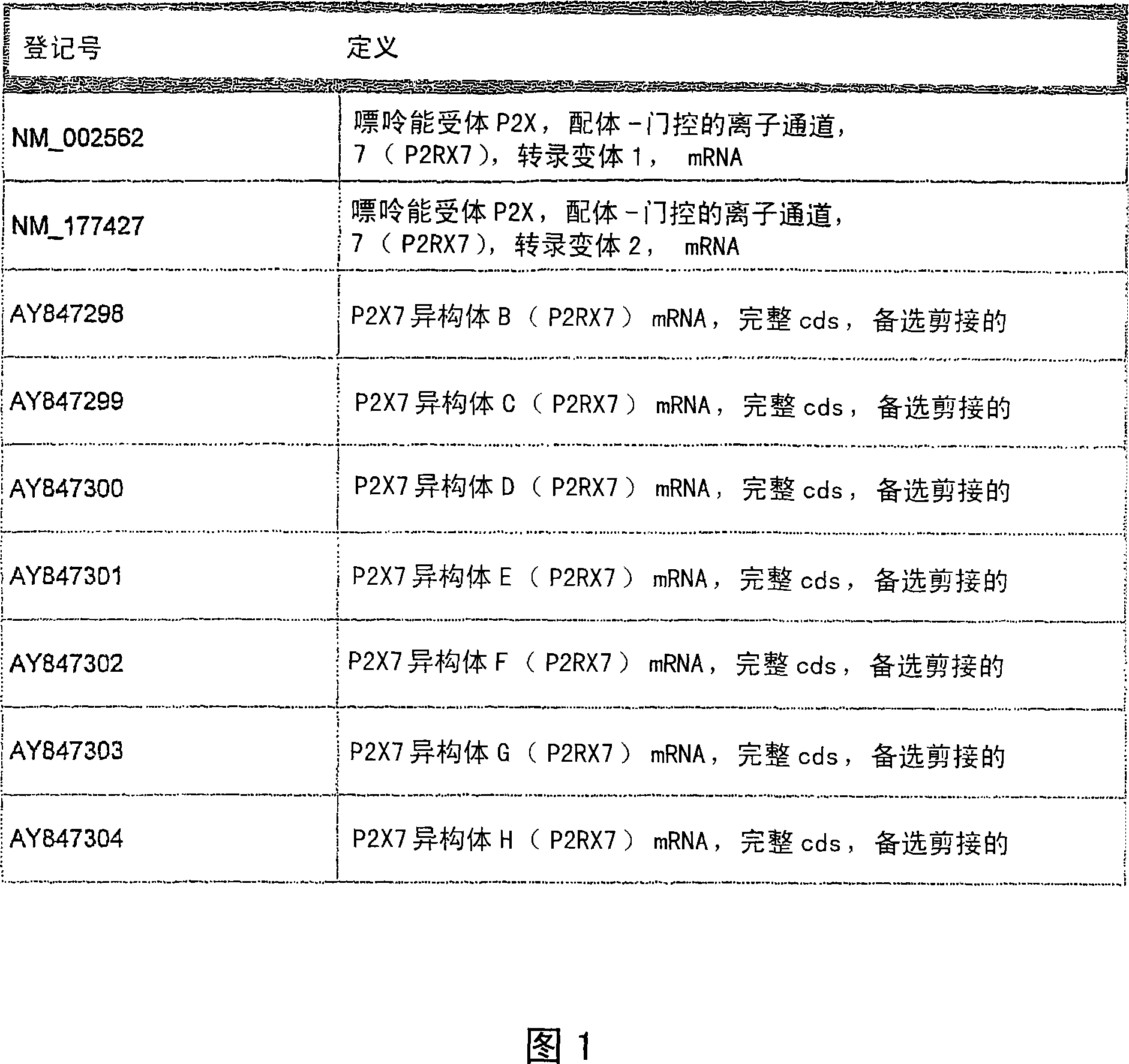
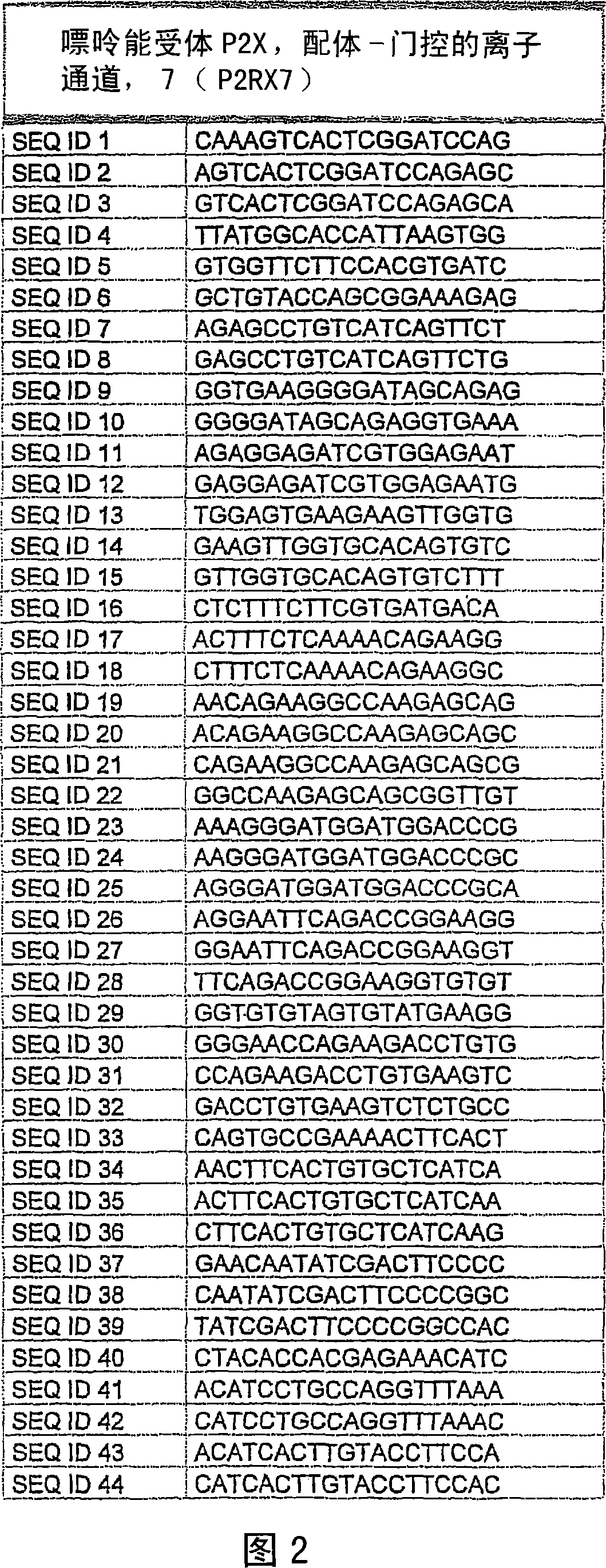
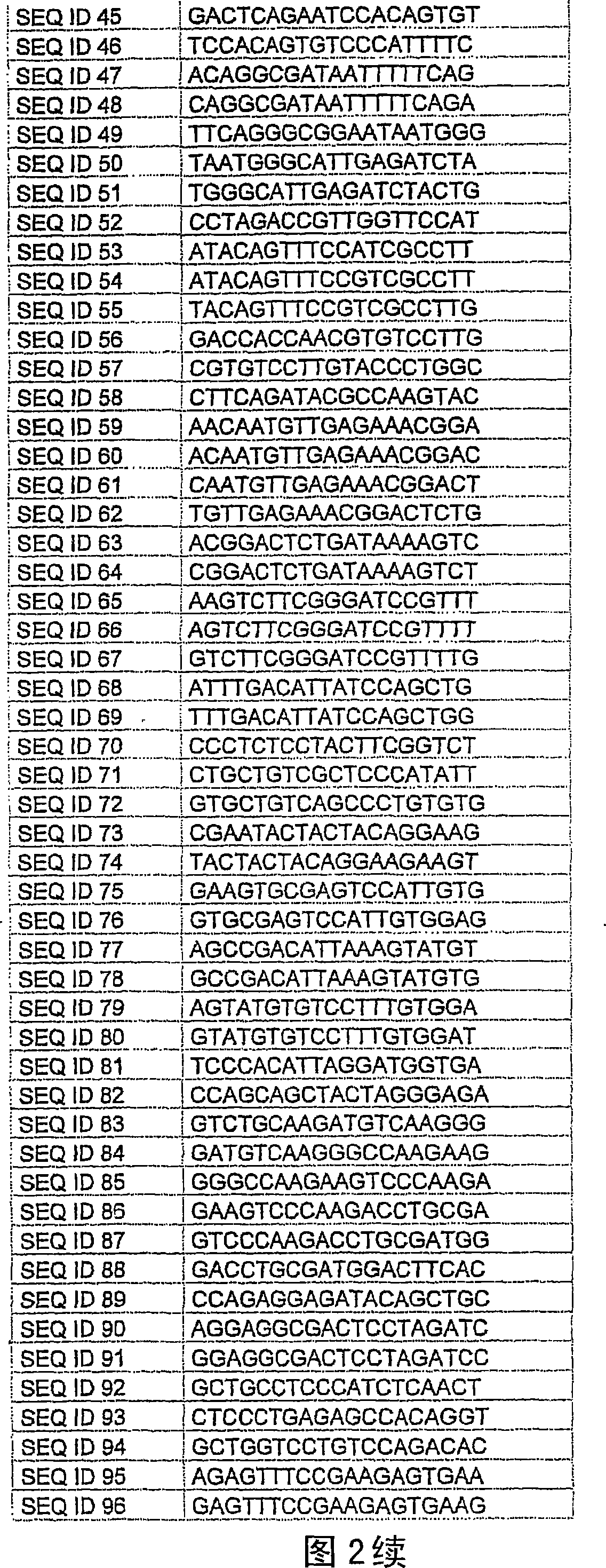
Methods and compositions for the downregulation of P2X7 receptor expression or activity are disclosed. Preferred compositions comprise siNA. The methods and compositions are useful in the treatment of diseases characterised by increased 112X7 receptor activity, such as neuronal degeneration, Alzheimer's disease, inflammatory diseases, and some cancers.
Owner:SYLENTIS
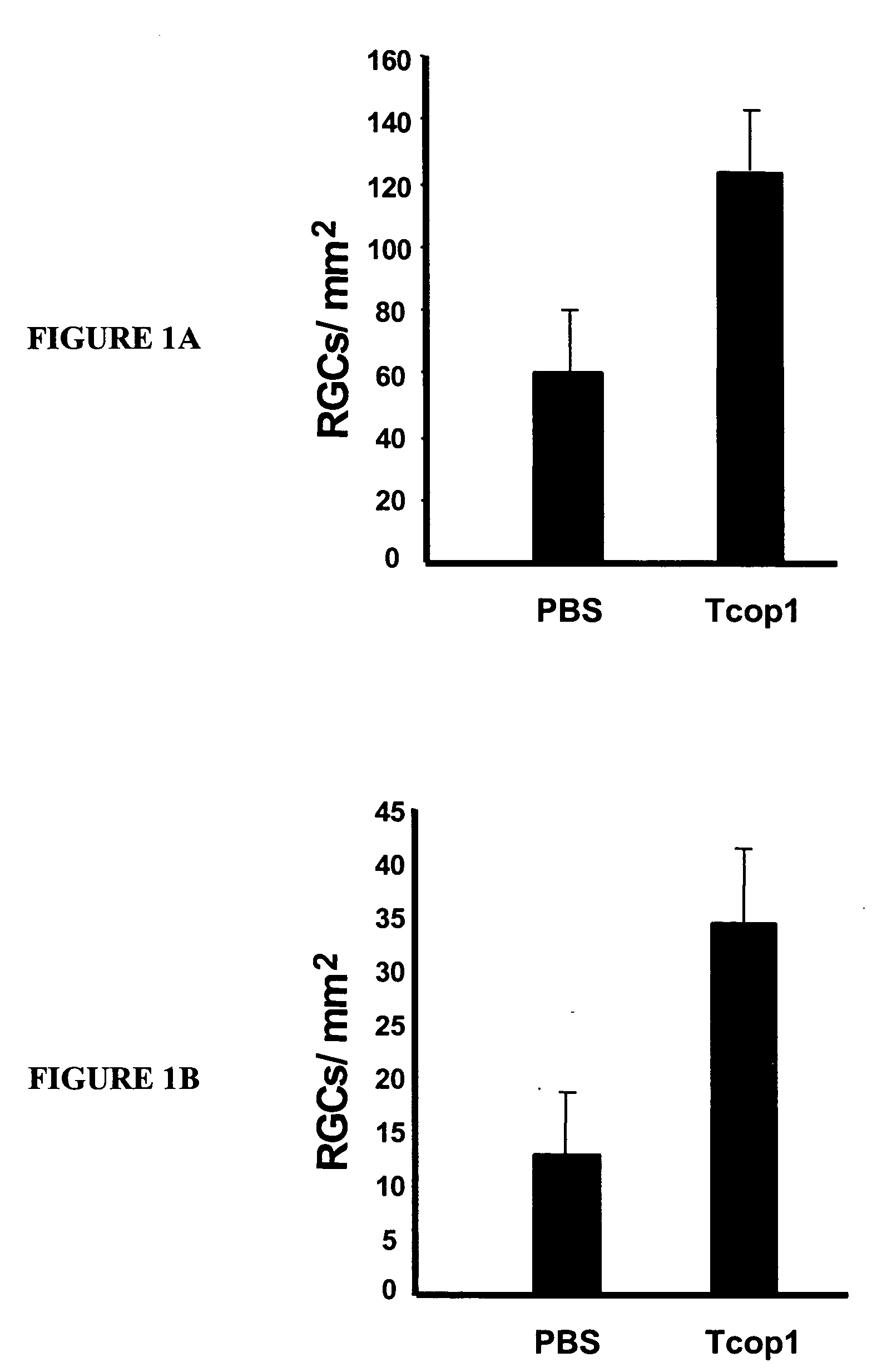
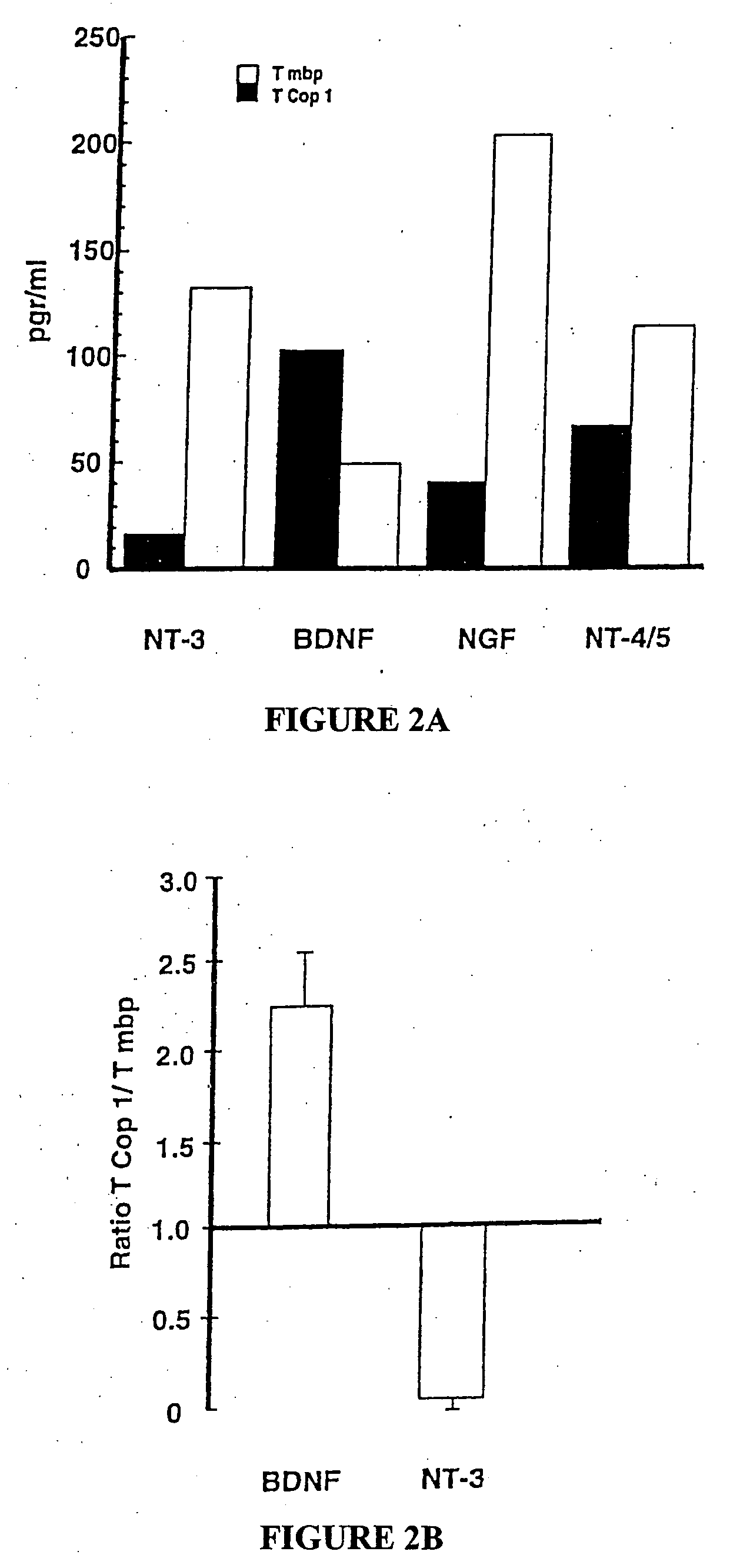
Use of copolymer 1 and related peptides and polypeptides and T cells treated therewith for neuroprotective therapy
Owner:YEDA RES & DEV CO LTD
Methods and Compositions to Inhibit P2x7 Receptor Expression
Methods and compositions for the downregulation of P2X7 receptor expression or activity are disclosed. Preferred compositions comprise siNA. The methods and compositions are useful in the treatment of diseases characterised by increased 112X7 receptor activity, such as neuronal degeneration, Alzheimer's disease, inflammatory diseases, and some cancers.
Owner:SYLENTIS
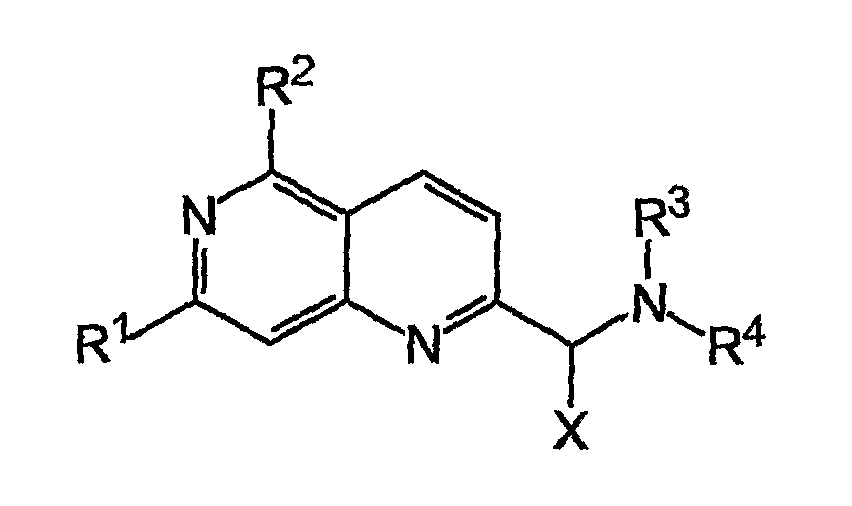
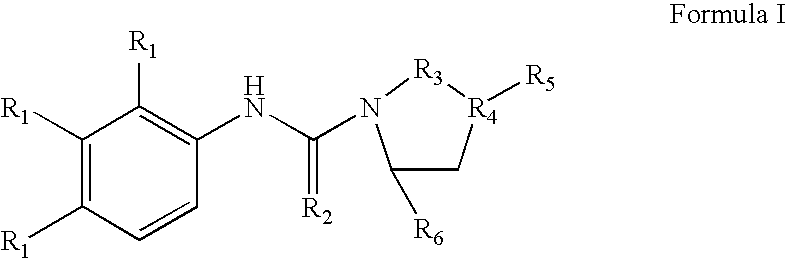
Napthyridine Compounds As Rock Inhibitors
InactiveUS20080207677A1Increase and stabilizes numberHigh activityBiocideSenses disorderLymphatic SpreadPercent Diameter Stenosis
The present invention relates to compounds having a naphthyridine scaffold, and stereoisomeric forms, prodrugs, solvates, hydrates and / or pharmaceutically acceptable salts of these compounds as well as pharmaceutical compositions containing at least one of these naphthyridine derivatives together with pharmaceutically acceptable carrier, excipient and / or diluents. Said naphthyridine compounds have been identified as inhibitors of the protein kinase ROCK2, also known as Rho-kinase, and are useful for the treatment of cancers (tumor growth and metastases), erectile dysfunction, cardiovascular diseases, hypertension, angina pectoris, cerebral ischaemia, cerebral vasospasm, myocardial ischaemia, coronary vasospasm, heart failure, myocardial hypertrophy, atherosclerosis, restenosis, spinal cord injuries, neuronal degeneration, thrombotic disorders, asthma, glaucoma, inflammation, anti-viral diseases (e.g. HIV), and osteoporosis.
Owner:GPC BIOTECH AG
Eye-drop vaccine containing copolymer 1 for therapeutic immunization
InactiveUS20090214470A1Promote nerve regenerationOrganic active ingredientsSenses disorderNervous systemNeuronal degeneration
The invention provides an eye-drop vaccine for therapeutic immunization of a mammal comprising Copolymer 1, a Copolymer 1-related peptide, or a Copolymer 1-related polypeptide, for treating neuronal degeneration caused by an injury or disease, disorder or condition in the central nervous system (CNS) or peripheral nervous system (PNS), for preventing or inhibiting neuronal secondary degeneration which may otherwise follow primary injury in the CNS, for promoting nerve regeneration in the CNS or in the PNS after an injury, disease, disorder or condition or for protecting CNS and PNS cells from glutamate toxicity.
Owner:YEDA RES & DEV CO LTD
Poly-Glu,Tyr for neuroprotective therapy
Methods and compositions are provided for preventing or inhibiting neuronal degeneration, or for promoting nerve regeneration, in the central nervous system (CNS) or peripheral nervous system (PNS), or for protecting nerves from glutamate toxicity, which comprises administering to an individual in need thereof an effective amount of the copolymer poly-Glu,Tyr.
Owner:YEDA RES & DEV CO LTD
Rotamase enzyme activity inhibitors
InactiveUS7056935B2Improve survivalPotent in augmenting neurite outgrowthBiocideOrganic chemistryNeuronal degenerationNeuron
This invention relates to methods of using neurotrophic compounds having an affinity for FKBP-type immunophilins to stimulate or promote neuronal growth or regeneration and to prevent neuronal degeneration.
Owner:GPI NIL HLDG INC +1
Construction method of glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell
InactiveCN103215225AViruses/bacteriophagesBiological testingGlial cell line-derived neurotrophic factorNeuronal degeneration
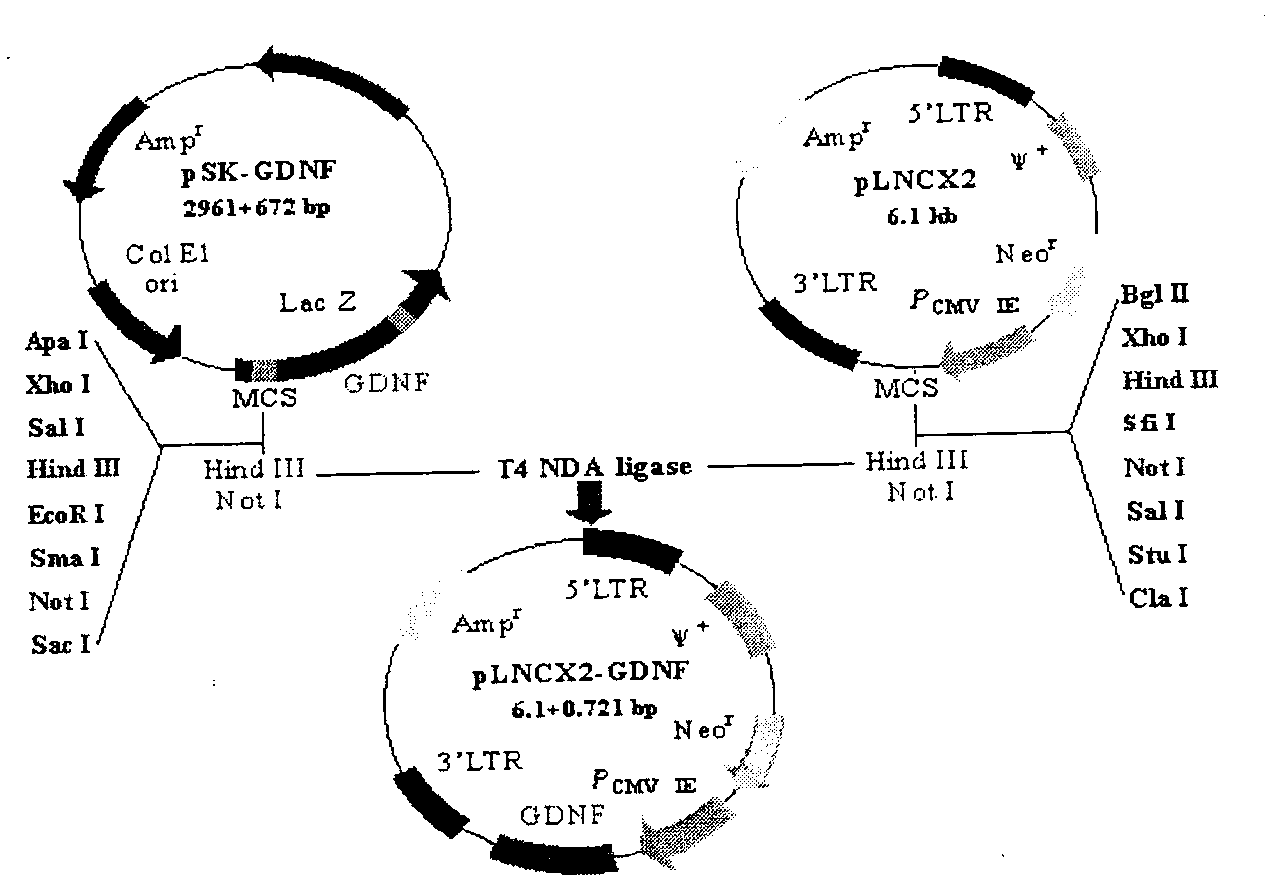
The invention relates to a biotechnology and especially relates to a construction method of a glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell. The construction method comprises the following steps of 1, plasmid transformation, 2, plasmid digestion purification, 3, target gene and vector cloning, 4, recombinant plasmid packaging, 5, recombinant plasmid identification, 6, virus titer determination, 7, stem cell culture, 8, virus infection of the stem cells, 9, transgenic stem cell identification, 10, transgenic stem cell target-protein content determination, and 11, transgenic stem cell biological-activity detection. The glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell has strong effects of promoting survival of dopaminergic neurons and retains characteristics of the original neural stem cell. The glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell has good effects of treating motor neuron damage diseases and neuronal degeneration diseases.
Owner:孙勇
T. cruzi-derived neurotrophic agents and methods of use therefor
InactiveUS7060676B2Provide supportNervous disorderPeptide/protein ingredientsNeuronal degenerationMammal
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TRUSTEES OF TUFTS COLLEGE
Synergistic Neuroprotective, Combinations Of Flavanols, Hydroxystilbenes, Flavanones, Flavones, Flavonols, Proanthocyanidins And Anthocyanidins
InactiveUS20080026086A1Delay and reduce and prevent age related decline in cognitive functionReduce or prevent age related decline in cognitive functionBiocideNervous disorderNeuronal degenerationProanthocyanidin
A synthetic composition is provided comprising (a) one or more compounds selected from the group consisting of flavanols and hydroxystilbenes; and (b) one or more compounds selected from the group consisting of flavanones, flavones, flavonols, proanthocyanidins and anthocyanidins. Also provided is a method of reducing neuronal degeneration in an individual which method comprises administering to the individual said composition.
Owner:CONOPCO INC D B A UNILEVER
Method for reducing neuronal degeneration so as to ameliorate the effects of injury or disease
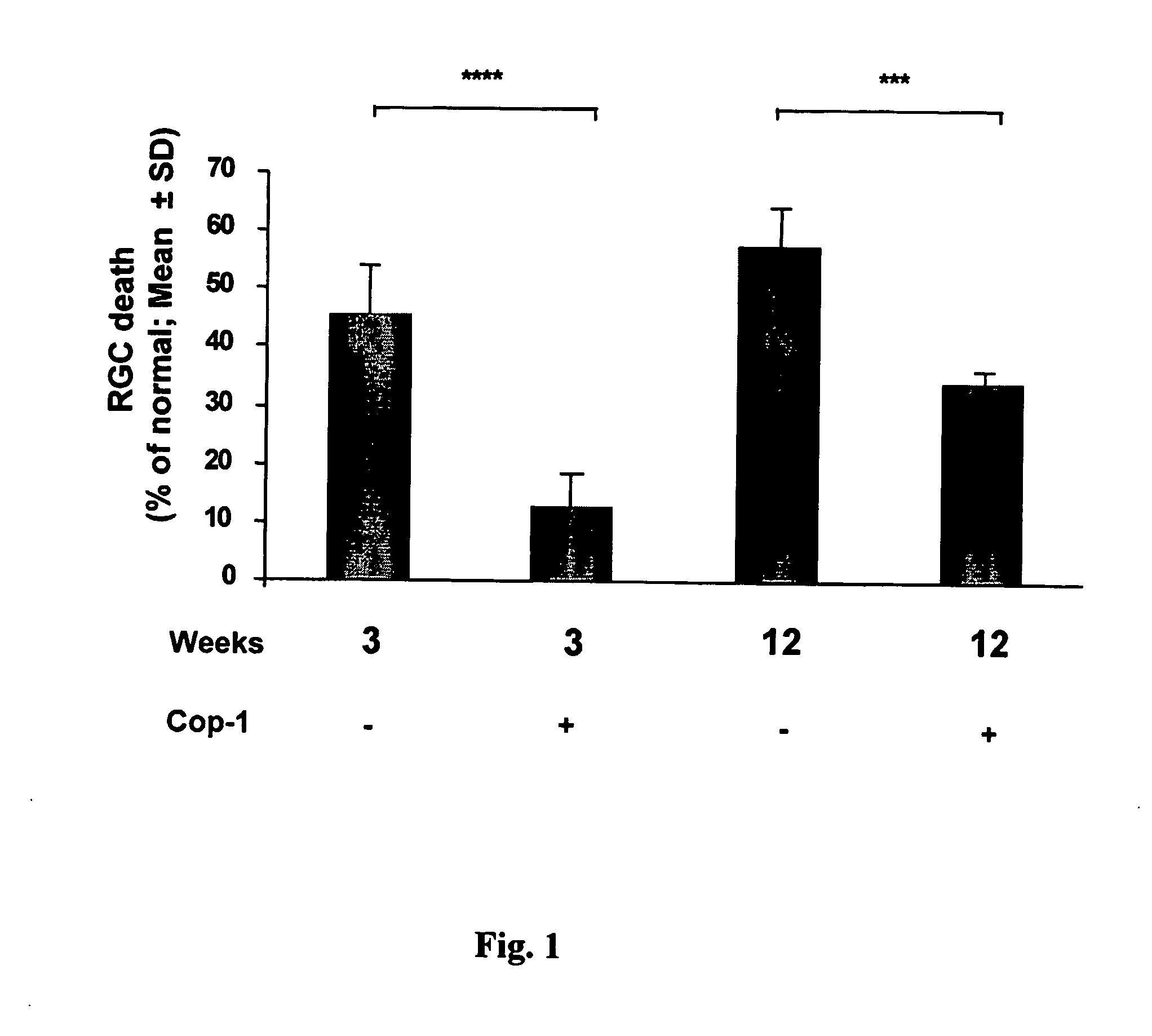
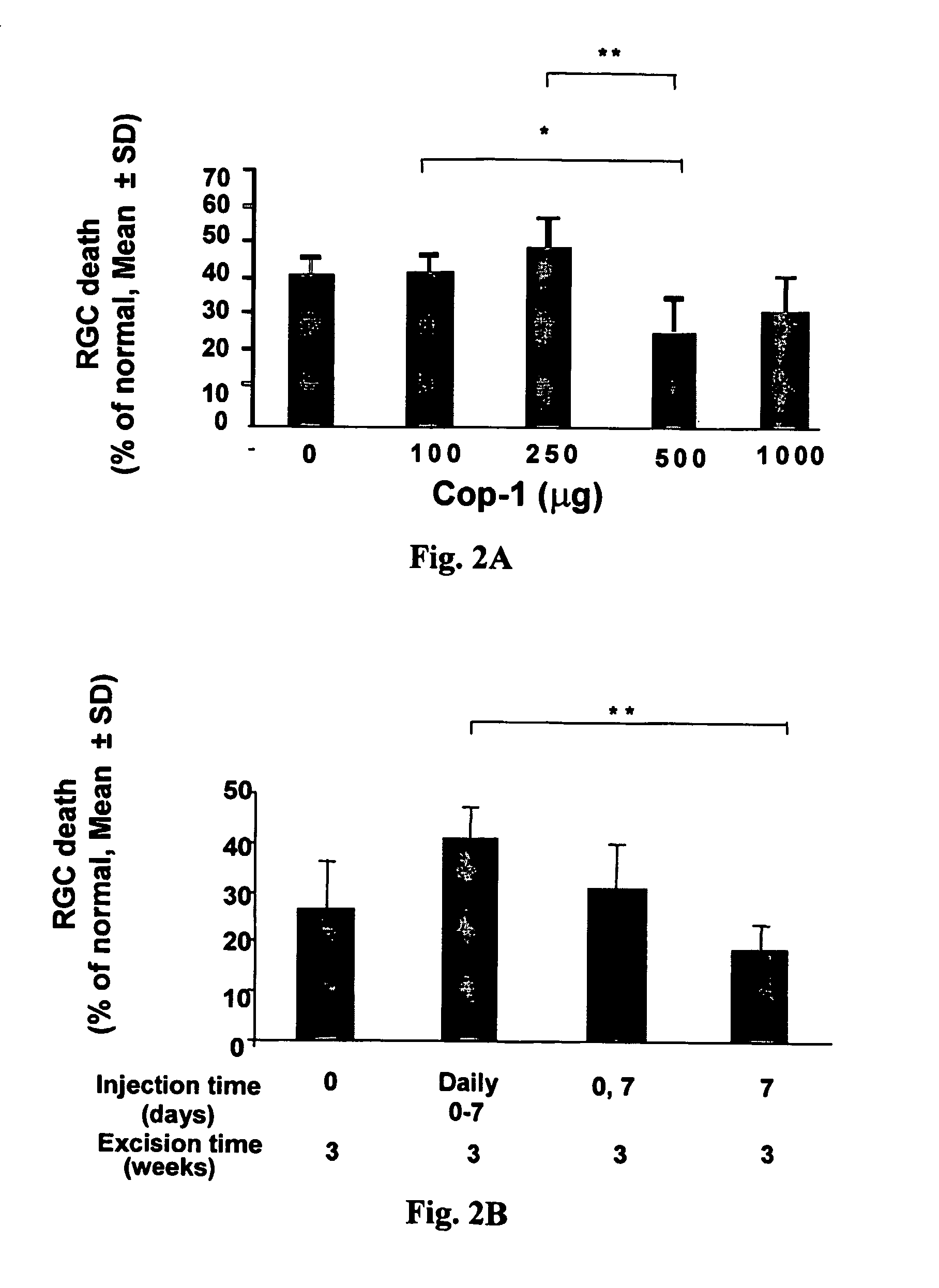
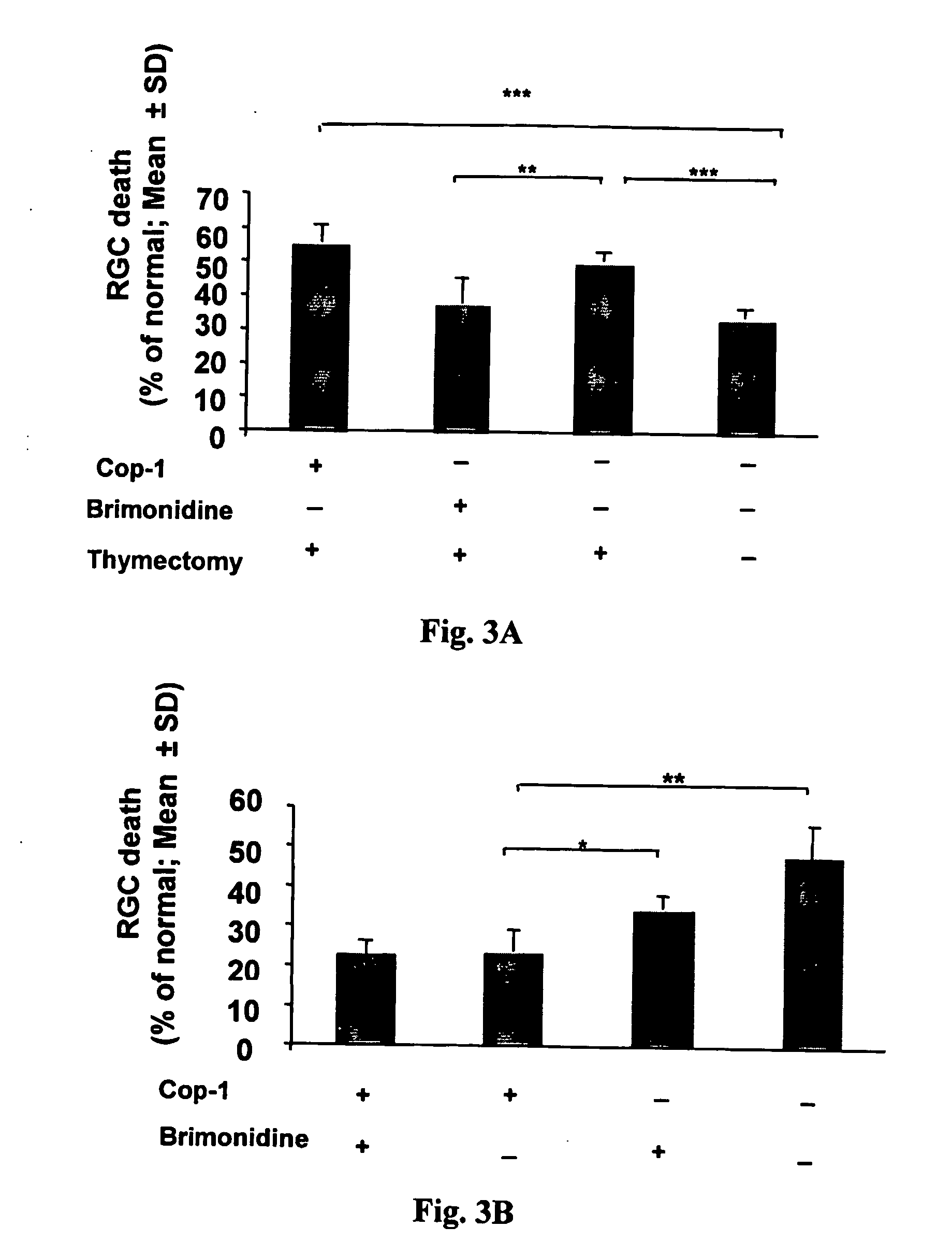
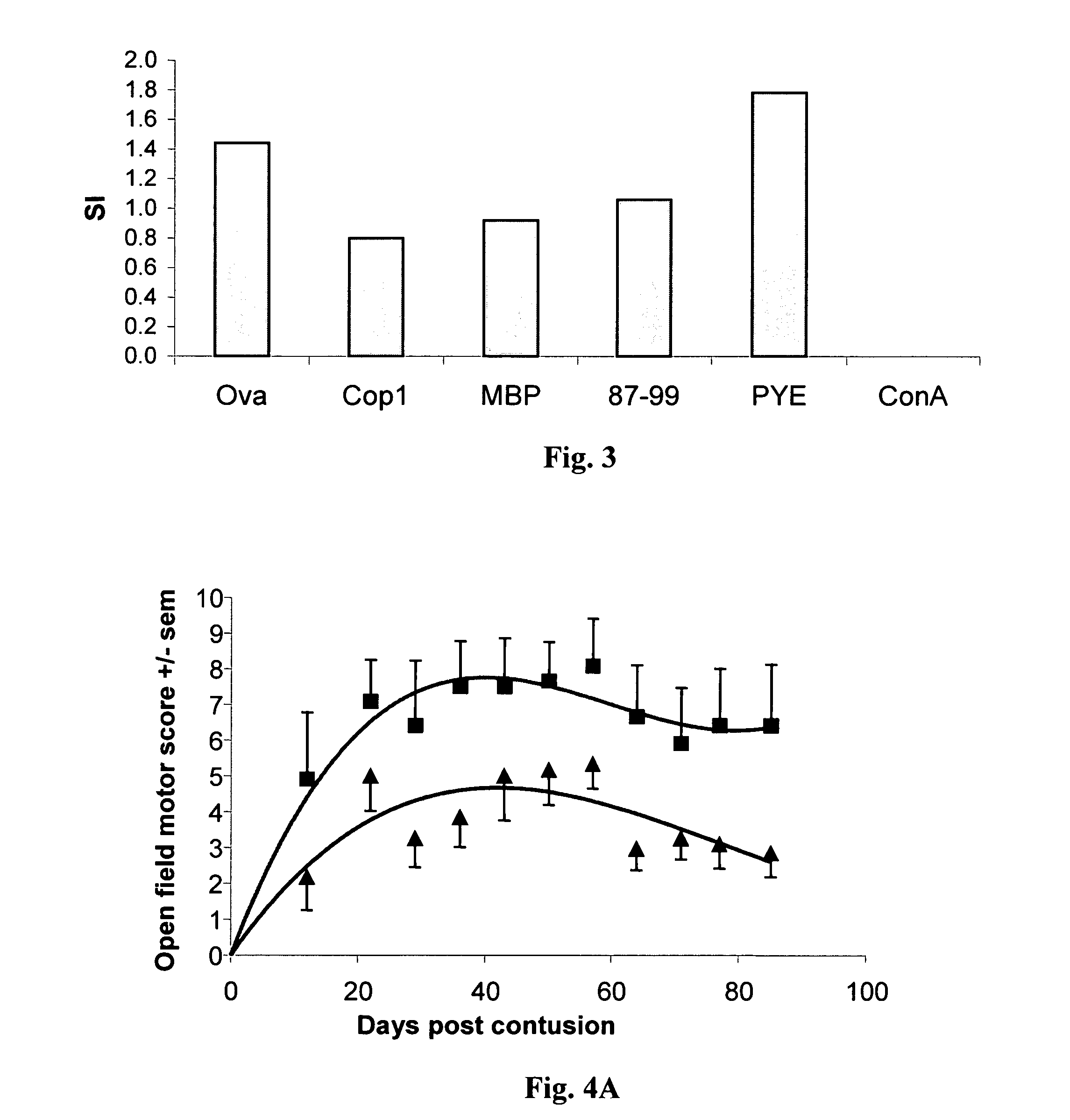
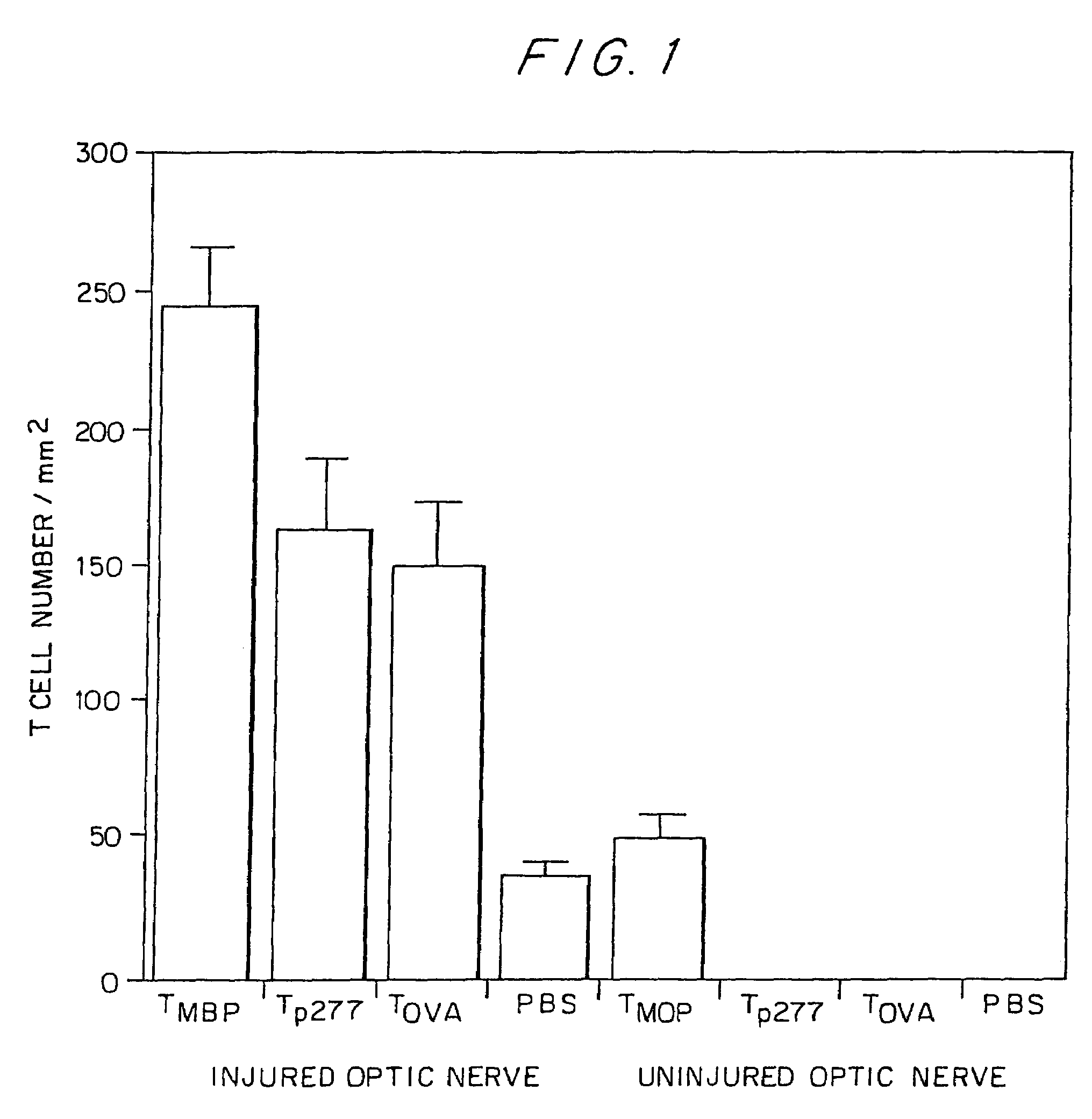
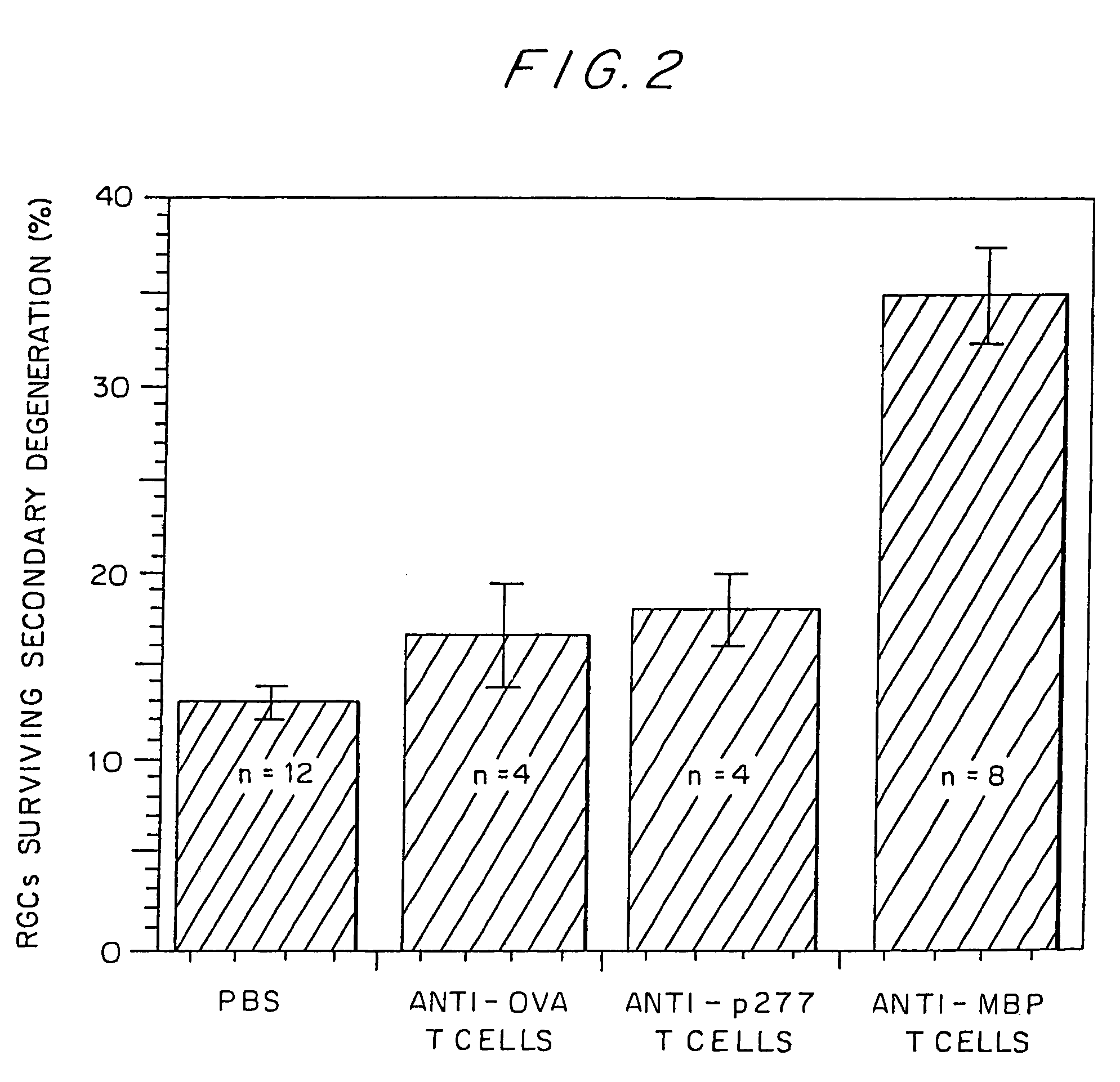
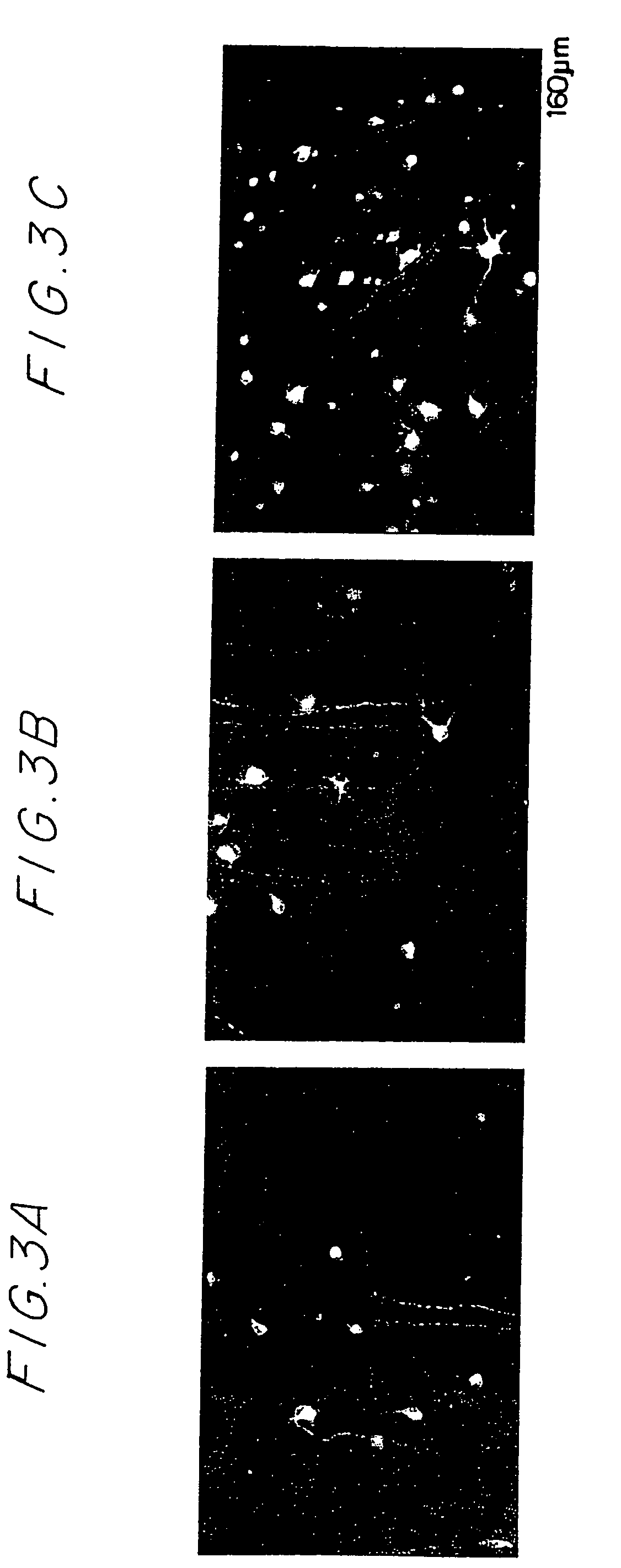
Compositions and methods to promote nerve regeneration or to confer neuroprotection and prevent or inhibit neuronal degeneration within the nervous system, either the central nervous system or the peripheral nervous system, are provided. Treatment involves administering NS-specific activated T cells, or an NS-specific antigen or analog thereof, a peptide derived therefrom or an analog or derivative of said peptide, or a nucleotide sequence encoding such an antigen or peptide, or any combination thereof.
Owner:YEDA RES & DEV CO LTD
Herbal composition for treatment of neuronal injuries and neuronal degeneration, methods to prepare the same and uses thereof
InactiveUS7205004B2Highly effective and economical and convenient treatmentBiocideNervous disorderNeuronal damageNeuronal degeneration
This invention provides a composition of herbs comprising Radix angelica sinensis (DangGui) 15–60%, Ligusticum chuanxiong (ChuanXiong) 5–20%, Hirudo (ShuiZhi) 3–7%, Polygonatum sibiricum (HuangJing) 4–15%. This invention further provides various uses of this composition.
Owner:XIA YONGCHAO
T. Cruzi-derived neurotrophic agents and methods of use therefor
InactiveUS20090117593A1Provide supportCompound screeningApoptosis detectionNeurophysinsNeuronal degeneration
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TUFTS UNIV
Neuronal Viability Factor and Use Thereof
This invention relates to methods and compositions for detection and treatment of neurodegenerative diseases. In particular, the invention relates to polypeptides that can protect against neuron degeneration, nucleic acid molecules that encode such polypeptides, and antibodies that recognize said polypeptides.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
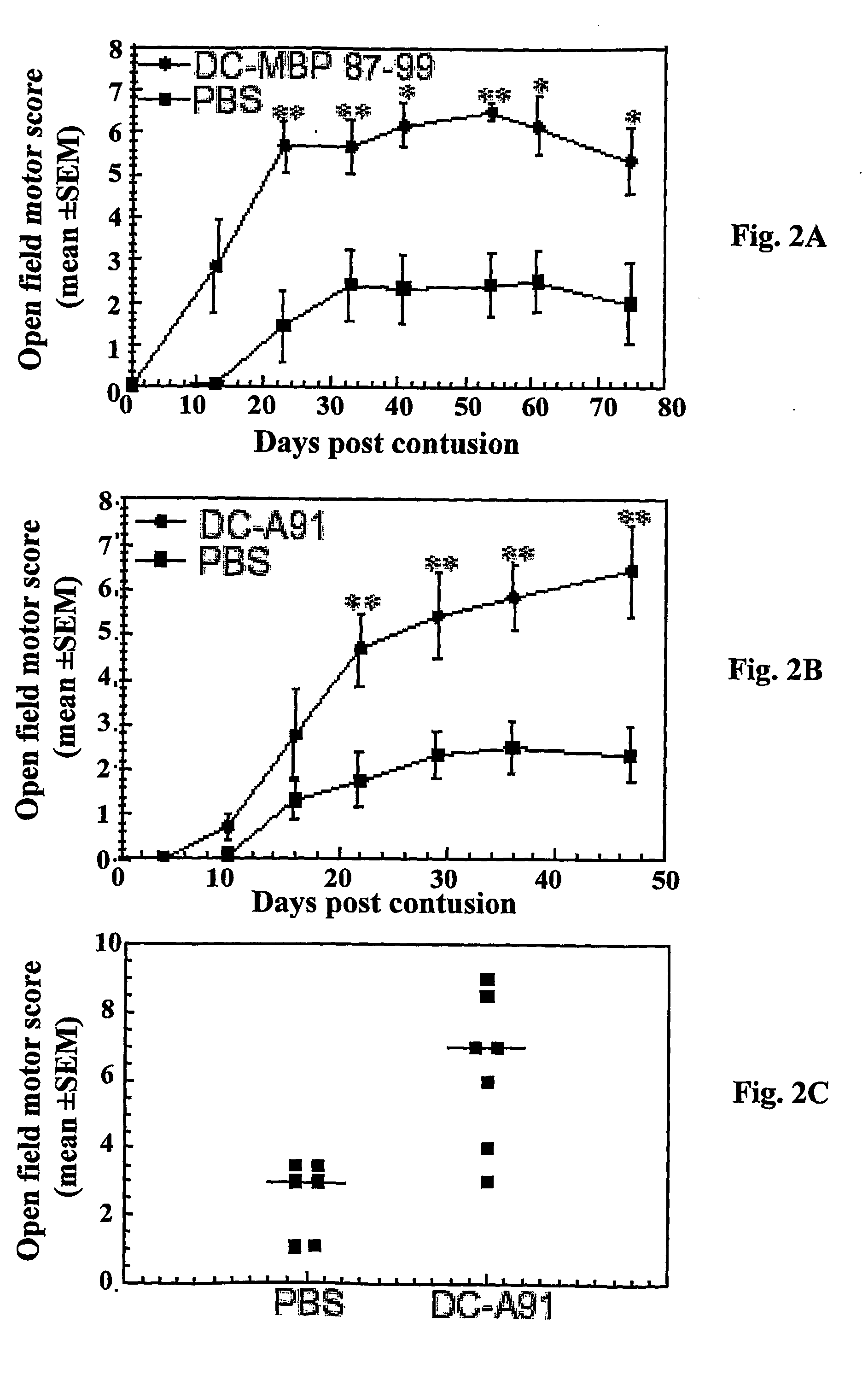
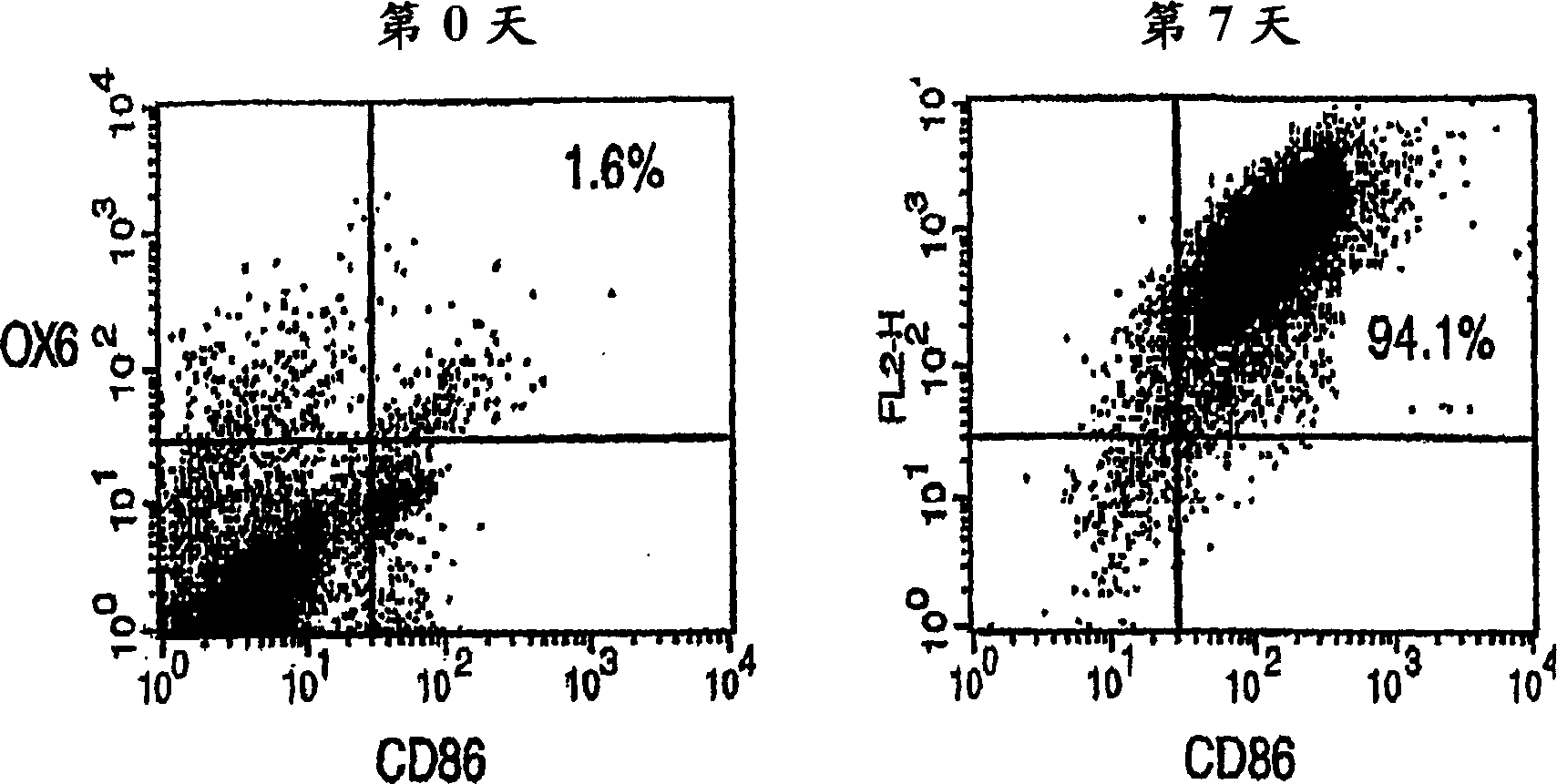
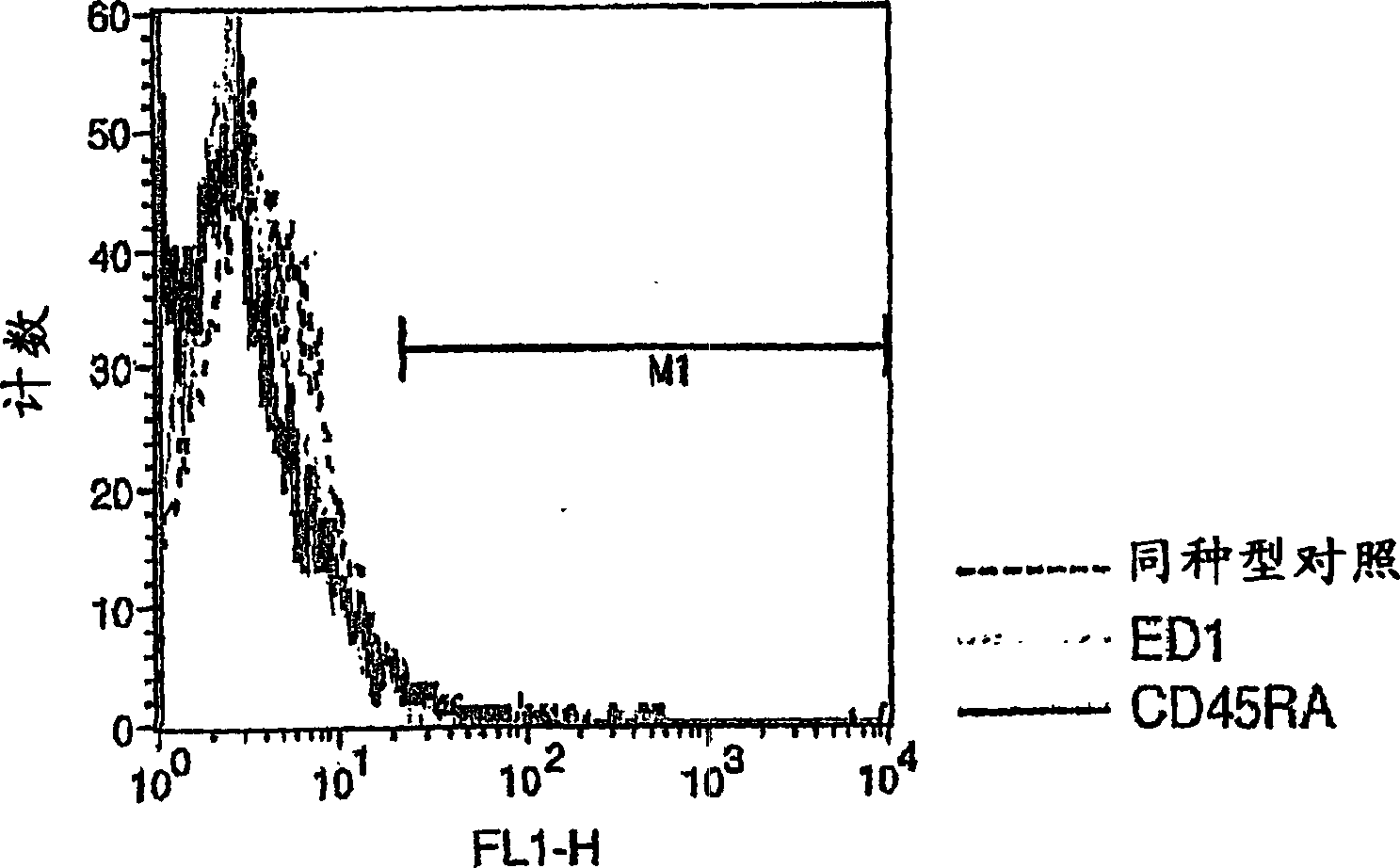
Antigen-presenting cells for neuroprotection and nerve regeneration
Pharmaceutical compositions and methods for preventing or inhibiting neuronal degeneration, or for promoting nerve regeneration, in the central nervous system (CNS) or peripheral nervous system (PNS), in the treatment of an injury, disorder or disease of the CNS or PNS, comprise antigen-presenting cells, preferably dendritic cells, that have been pulsed with an agent selected from the group consisting of: (a) a nervous system (NS)-specific antigen or an analog thereof; (b) a peptide derived from an NS-specific antigen or from an analog thereof, or an analog or derivative of said peptide; (c) a copolymer selected from the group consisting of Copolymer 1, a Copolymer 1-related peptide or polypeptide, and poly-Glu<50> Tyr<50>; and (d) a non-self antigen.
Owner:YEDA RES & DEV CO LTD
Antigen-presenting cells for neuroprotection and nerve regeneration
Pharmaceutical compositions and methods for preventing or inhibiting neuronal degeneration, or for promoting nerve regeneration, in the central nervous system (CNS) or peripheral nervous system (PNS), in the treatment of an injury, disorder or disease of the CNS or PNS, comprise antigen-presenting cells, preferably dendritic cells, that have been pulsed with an agent selected from the group consisting of: (a) a nervous system (NS)-specific antigen or an analog thereof; (b) a peptide derived from an NS-specific antigen or from an analog thereof, or an analog or derivative of said peptide; (c) a copolymer selected from the group consisting of Copolymer 1, a Copolymer 1-related peptide or polypeptide, and poly-Glu<50> Tyr<50>; and (d) a non-self antigen.
Owner:YEDA RES & DEV CO LTD
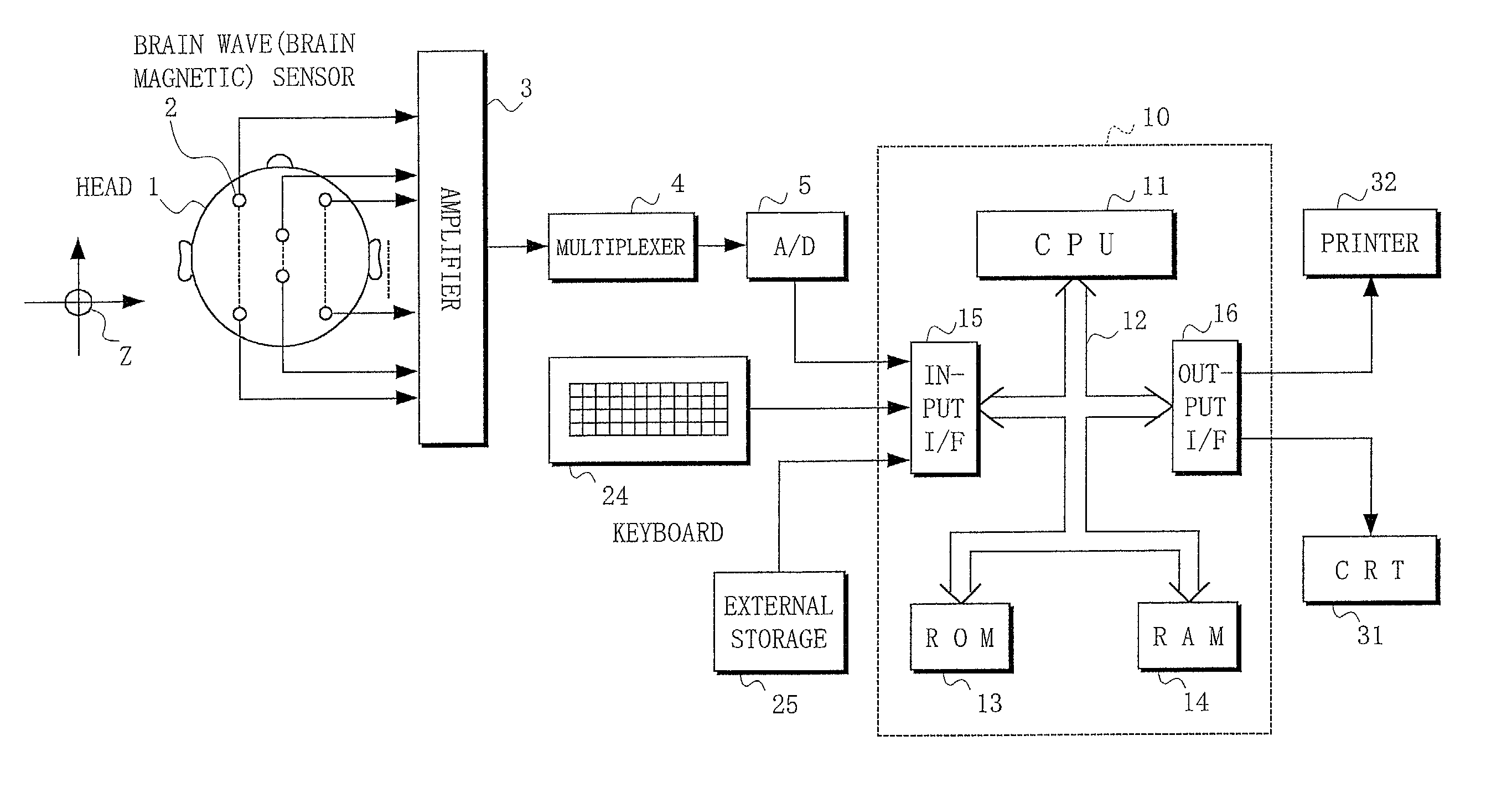
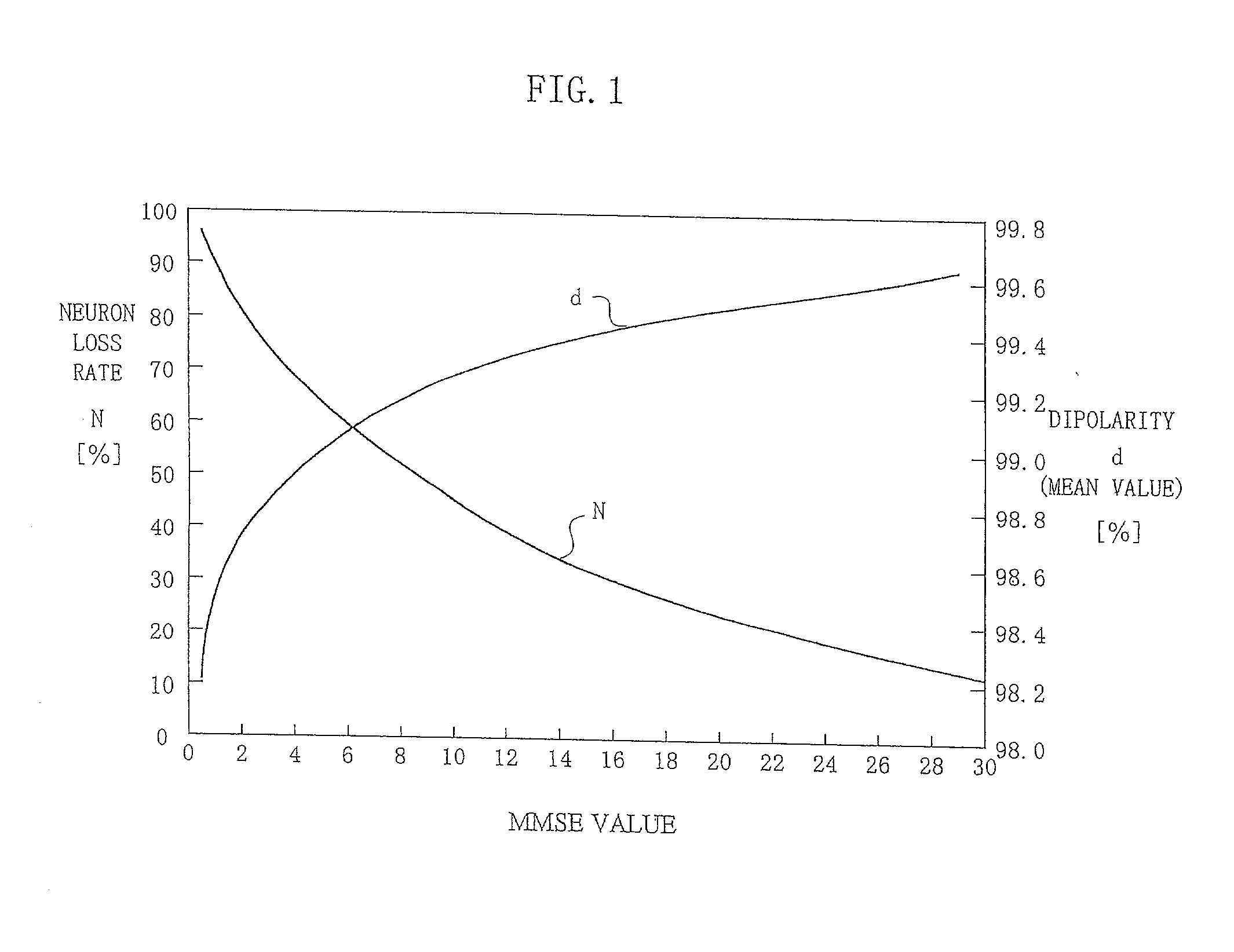
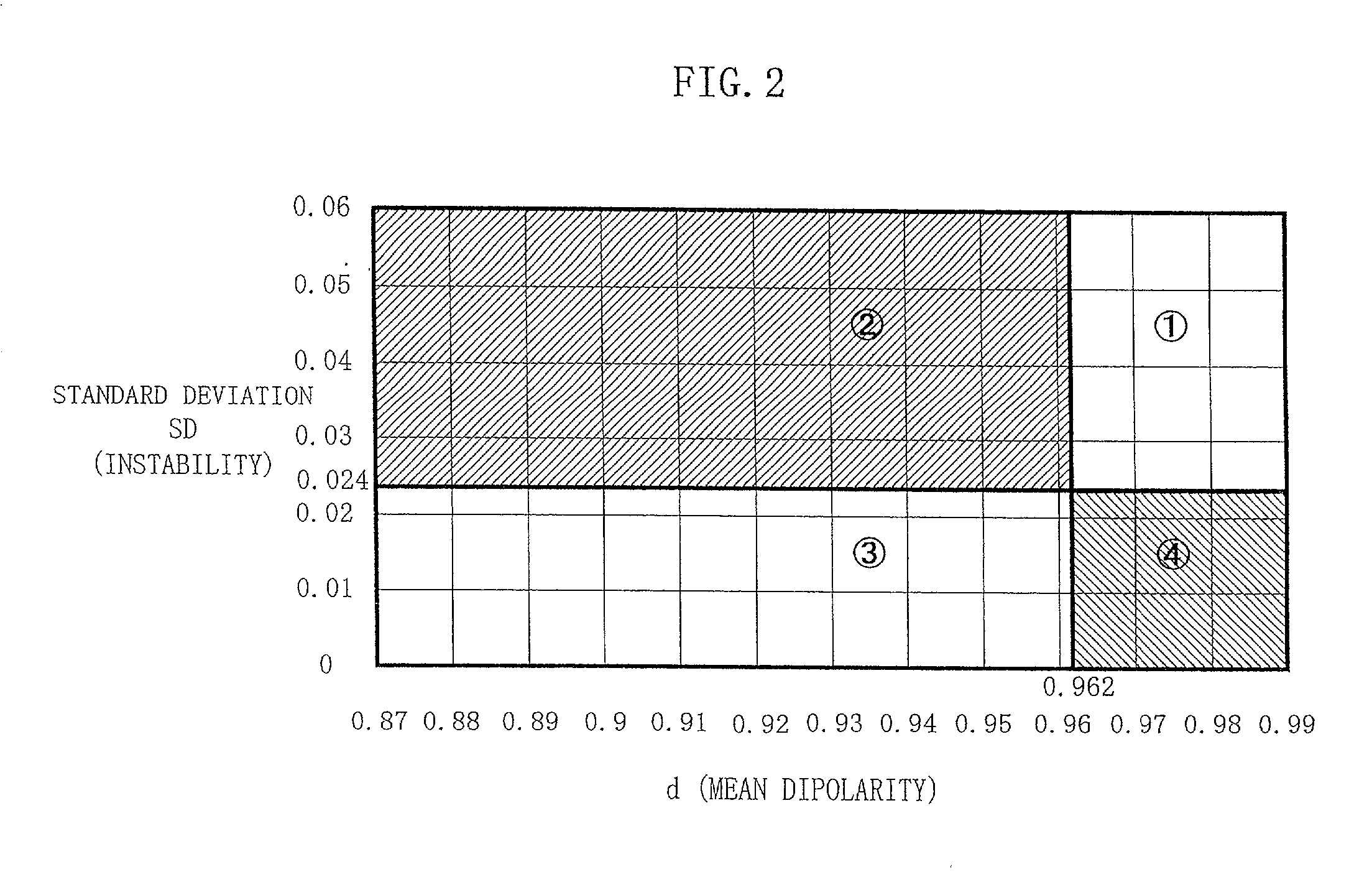
Method and apparatus for estimating degree of neuronal impairment in brain cortex
InactiveUS20020107455A1Improve reliabilityHigh sensitivityElectroencephalographySensorsNeuronal damageNeuronal degeneration
The neuronal activity in the brain of demented subject becomes non-uniform over the cortical surface and this non-uniformity is sensitively detected by properly analyzing observed scalp potentials. The present invention is related to the computer algorithm and apparatus for numerically detecting the degree of such neuronal degeneration. A proper frequency component of EEG is selected so that its scalp potential becomes smooth in a normal subject. When the brain function is impaired, non-uniformity of neuronal activity is detected in spatial as well as temporal fluctuations in the observed scalp potential of properly filtered EEG (usually the alpha component). The statistical parameters derived from dipolarity values of such a properly selected EEG component determine the state of the brain, in which whether the subject is normal or demented is Judged in the early stage of dementia by comparing the obtained parameters with their threshold values. This invention presents a reliable, inexpensive, easy-to-handle and non-invasive method for sensitive screening of dementia and monitoring progress of dementia which also allows optimizing medication and treatment for dementia.
Owner:BRAIN FUNCTIONS LAB
Transgenic Drosophila having a disrupted Parkin gene and exhibits reduced climbing ability
InactiveUS6943278B2LigasesVector-based foreign material introductionNeuronal degenerationNeuronal disease
The present invention relates to animal models for neuronal function, e.g., spontaneous changes in motor ability related to neuronal degeneration. For example, the present invention provides a Drosophila melanogaster model for Parkinson's disease. The present invention also provides methods for generating genetically based neuronal disease models, identifying genes that affect neuronal function and methods for identifying compounds having activity with respect to neurological function.
Owner:GENEXEL
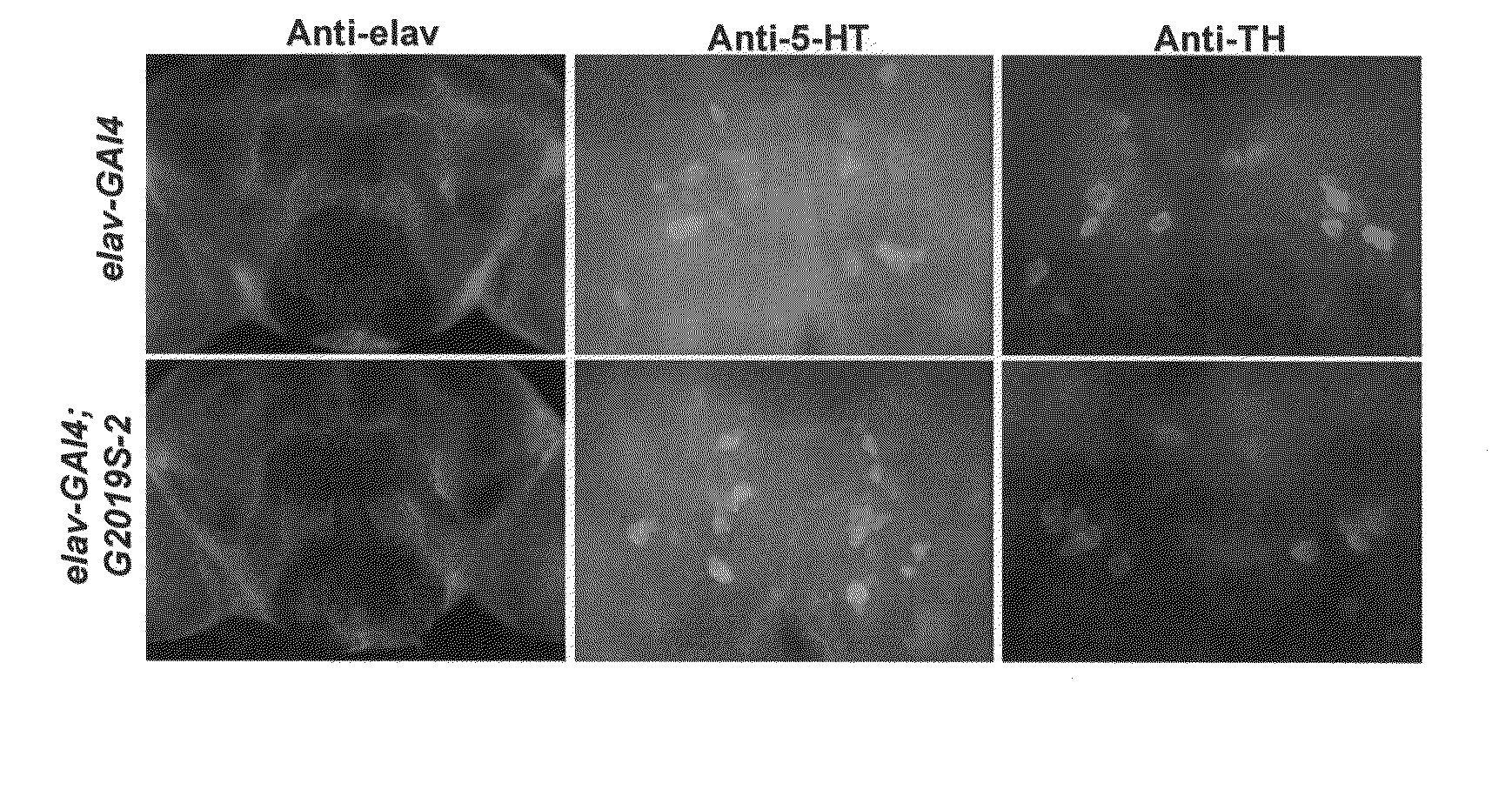
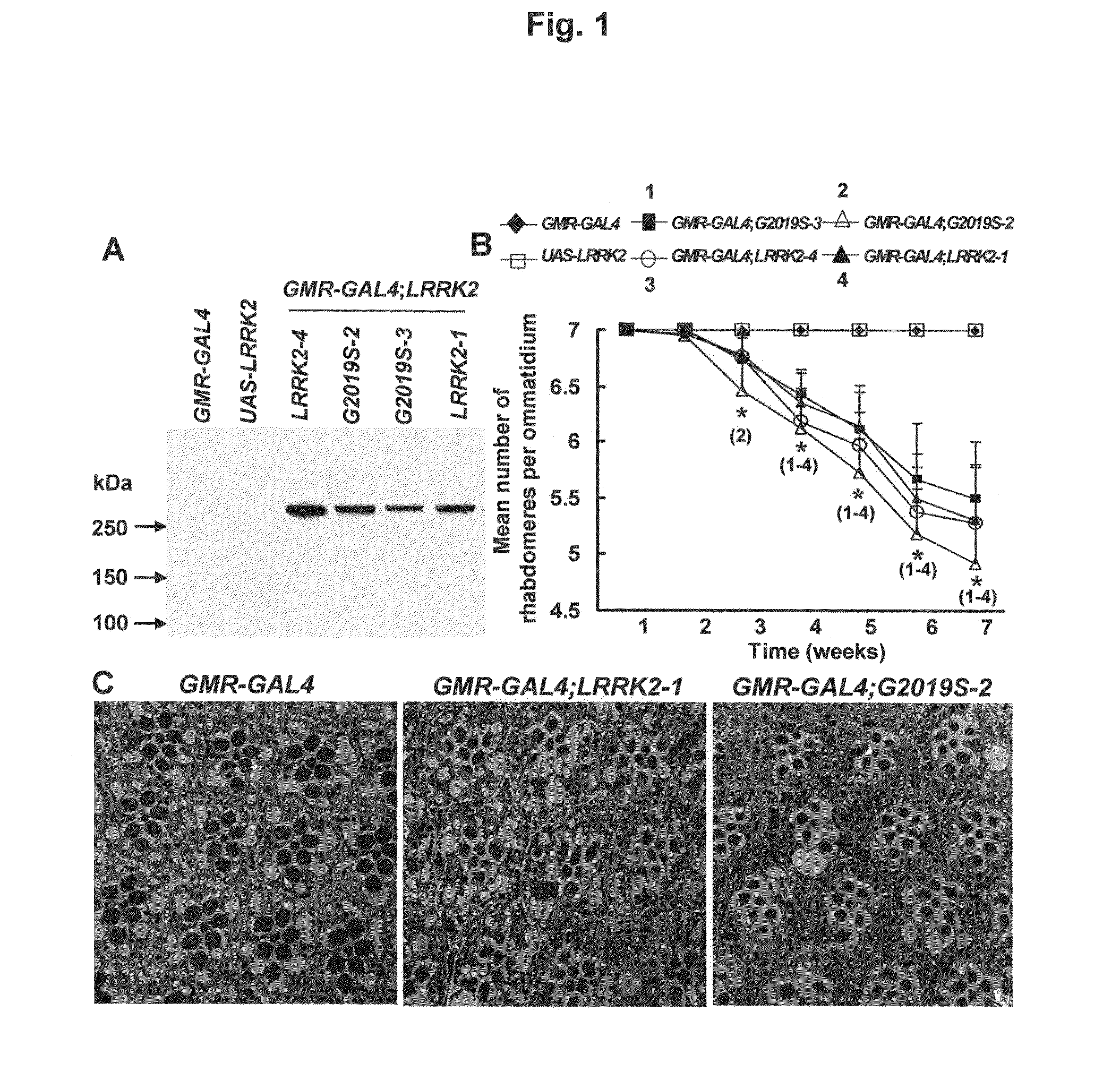
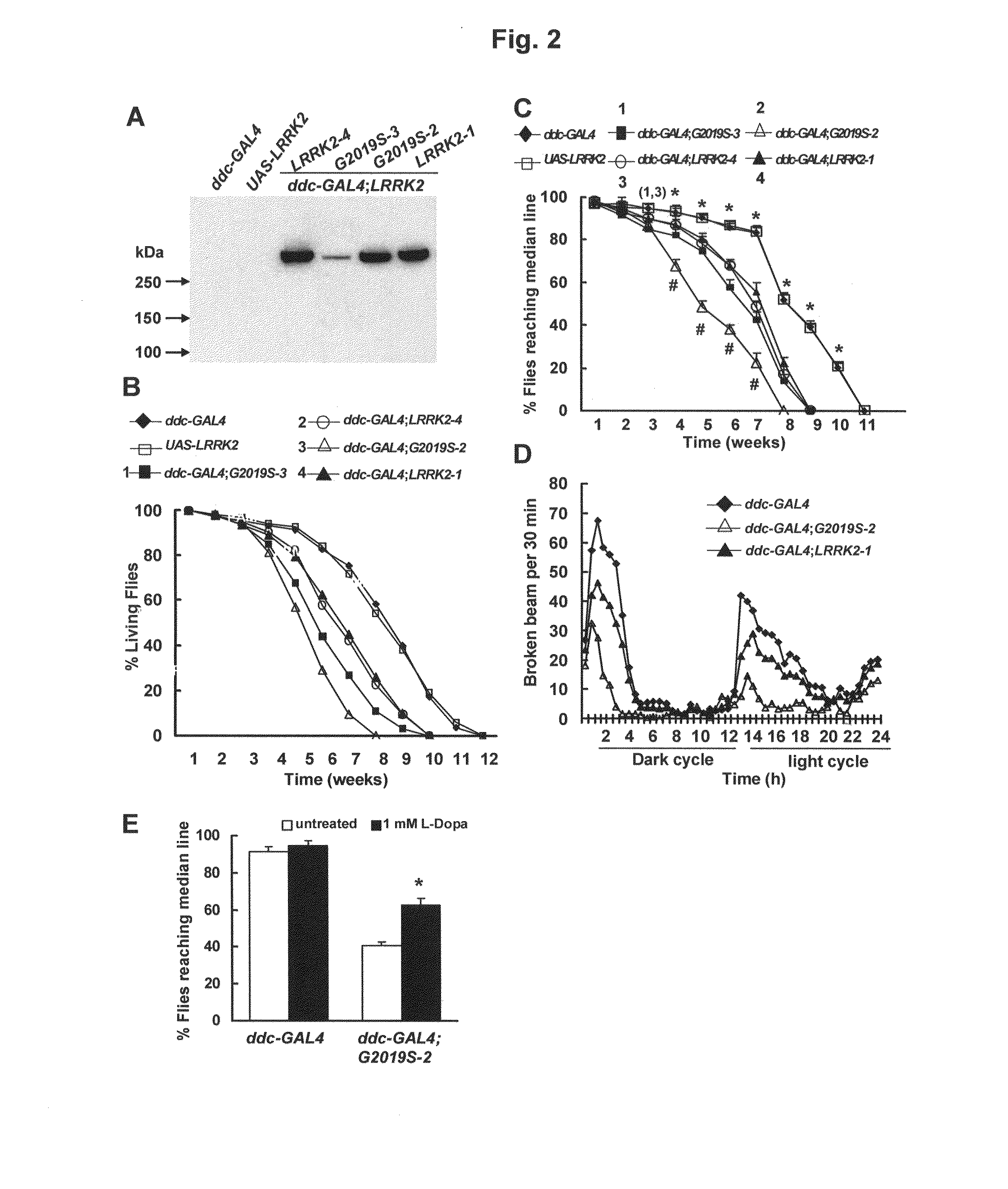
Leucine-rich repeat kinase (LRRK2) drosophila model for parkinson's disease: wildtype1 (WT1) and G2019S mutant flies
InactiveUS20100175140A1Effective preventionEffective treatmentCompounds screening/testingGenetic engineeringNeuronal degenerationWild type
Mutations in the leucine-rich repeat kinase (LRRK2) gene cause late-onset autosomal dominant Parkinson's disease (PD) with pleiomorphic pathology. Previously, we and others found that expression of mutant LRRK2 causes neuronal degeneration in cell culture. Here we used the GAL4 / UAS system to generate transgenic Drosophila expressing either wild-type (WT1) human LRRK2 or LRRK2-G2019S, the most common mutation associated with PD. Expression of either WT1 human LRRK2 or LRRK2-G2019S in the photoreceptor cells caused retinal degeneration. Expression of WT1 LRRK2 or LRRK2-G2019S in neurons produced adult-onset selective loss of dopaminergic neurons, locomotor dysfunction, and early mortality. Expression of mutant G2019S-LRRK2 caused a more severe parkinsonism-like phenotype than expression of equivalent levels of WT1 LRRK2. Treatment with L-DOPA improved mutant LRRK2-induced locomotor impairment but did not prevent the loss of tyrosine hydroxylase (TH)-positive neurons. To our knowledge, this is the first in vivo “gain-of-function” model which recapitulates several key features of LRRK2-linked human parkinsonism. These flies may provide a useful model for studying LRRK2-linked pathogenesis and for future therapeutic screens for PD intervention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
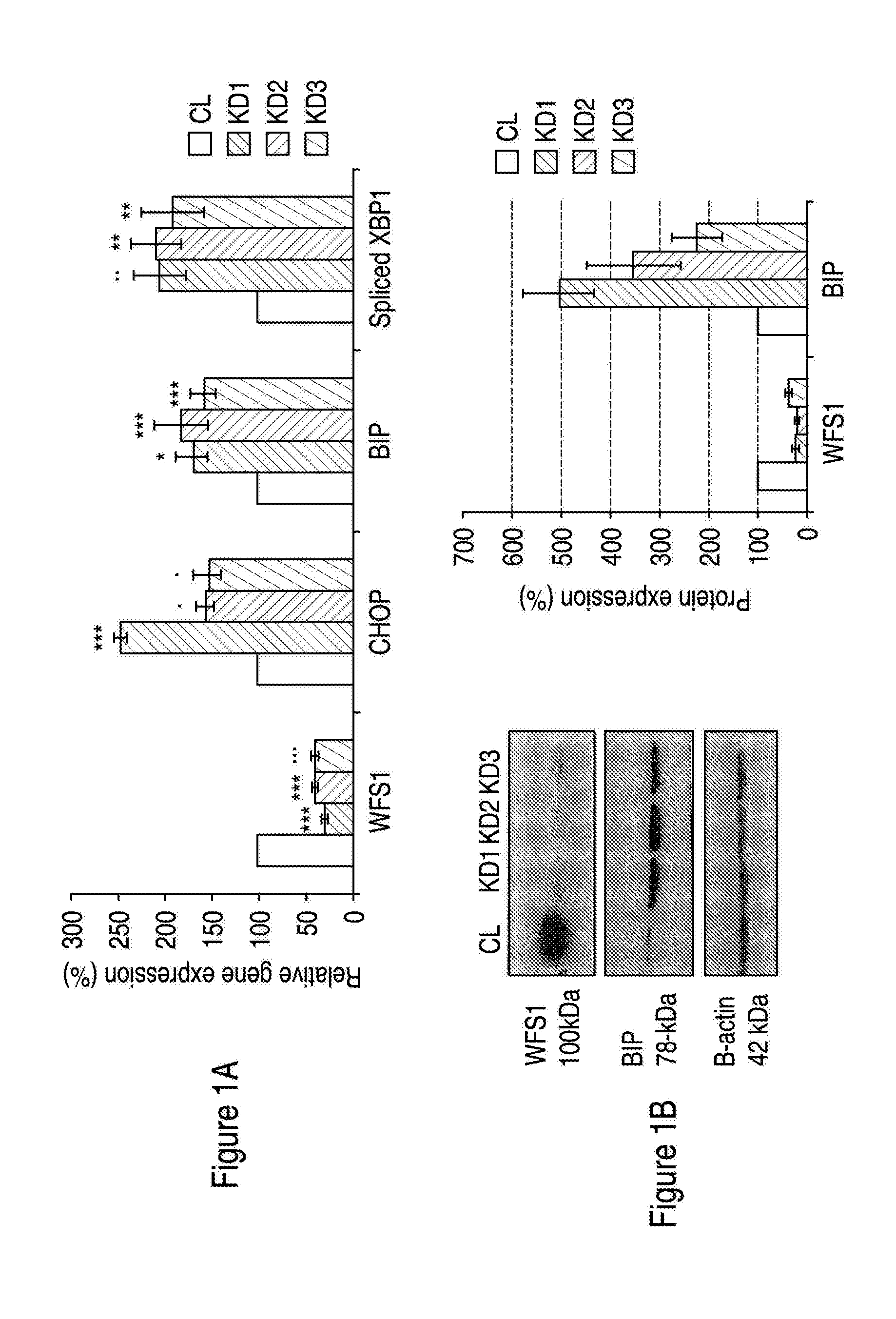
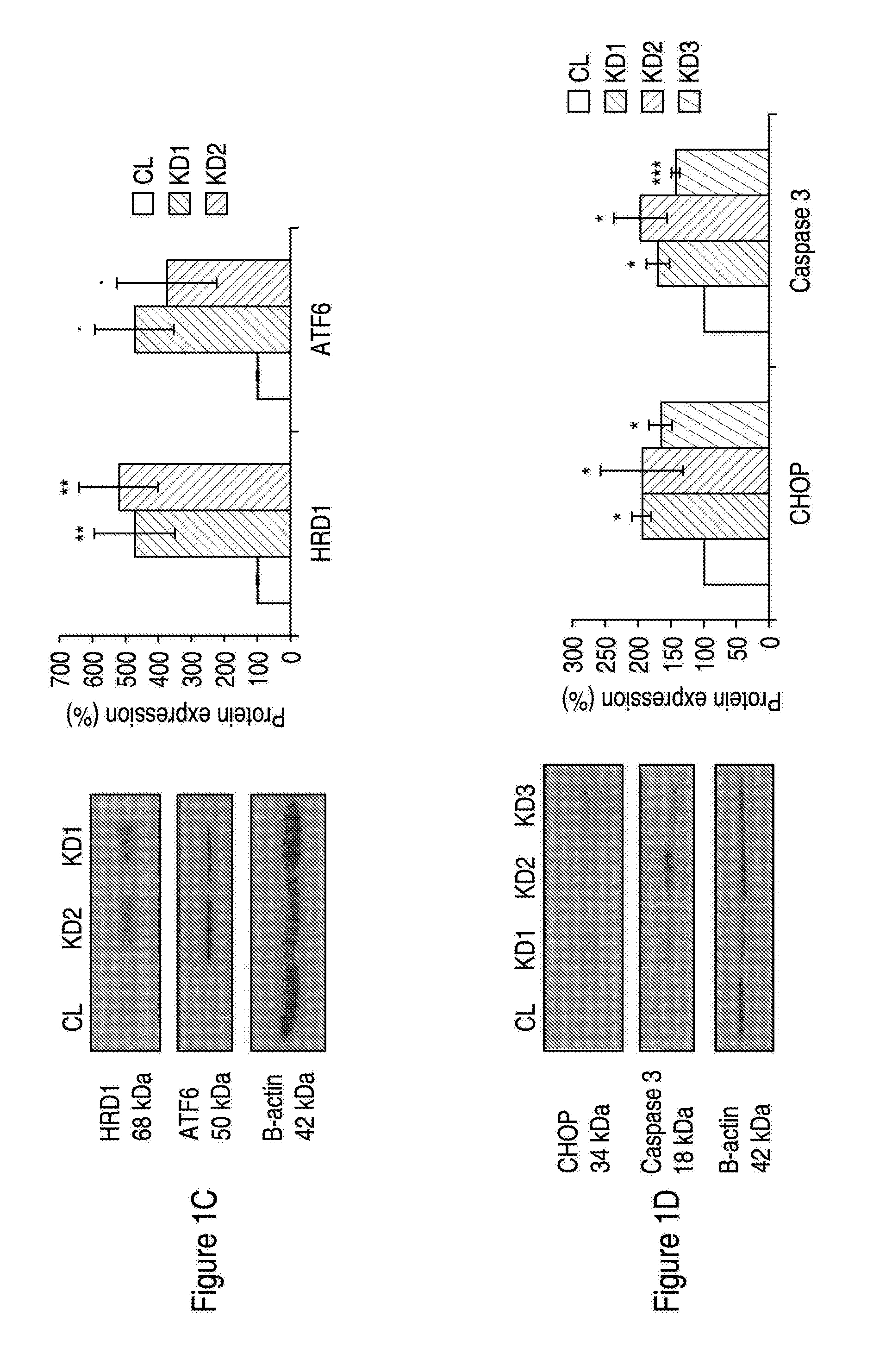
Treatment for neurodegeneration
ActiveUS20150246037A1Avoid cell deathInhibiting neurodegenerationBiocideNervous disorderNeuronal degenerationFunctional activity
Provided is a method of treating and / or preventing Wolfram Syndrome (WS)-related neurodegeneration (i.e. of Wolfram Syndrome-Associated Neuronal Degeneration), by increasing the expression and / or functional activity of p21. A method of screening for pharmacological agents useful in treating and / or preventing such conditions is also provided.
Owner:THE UNIV OF BIRMINGHAM
T. cruzi-derived neurotrophic agents and methods of use therefor
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TRUSTEES OF TUFTS COLLEGE
T. cruzi-derived neurotrophic agents and methods of use therefor
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TRUSTEES OF TUFTS COLLEGE
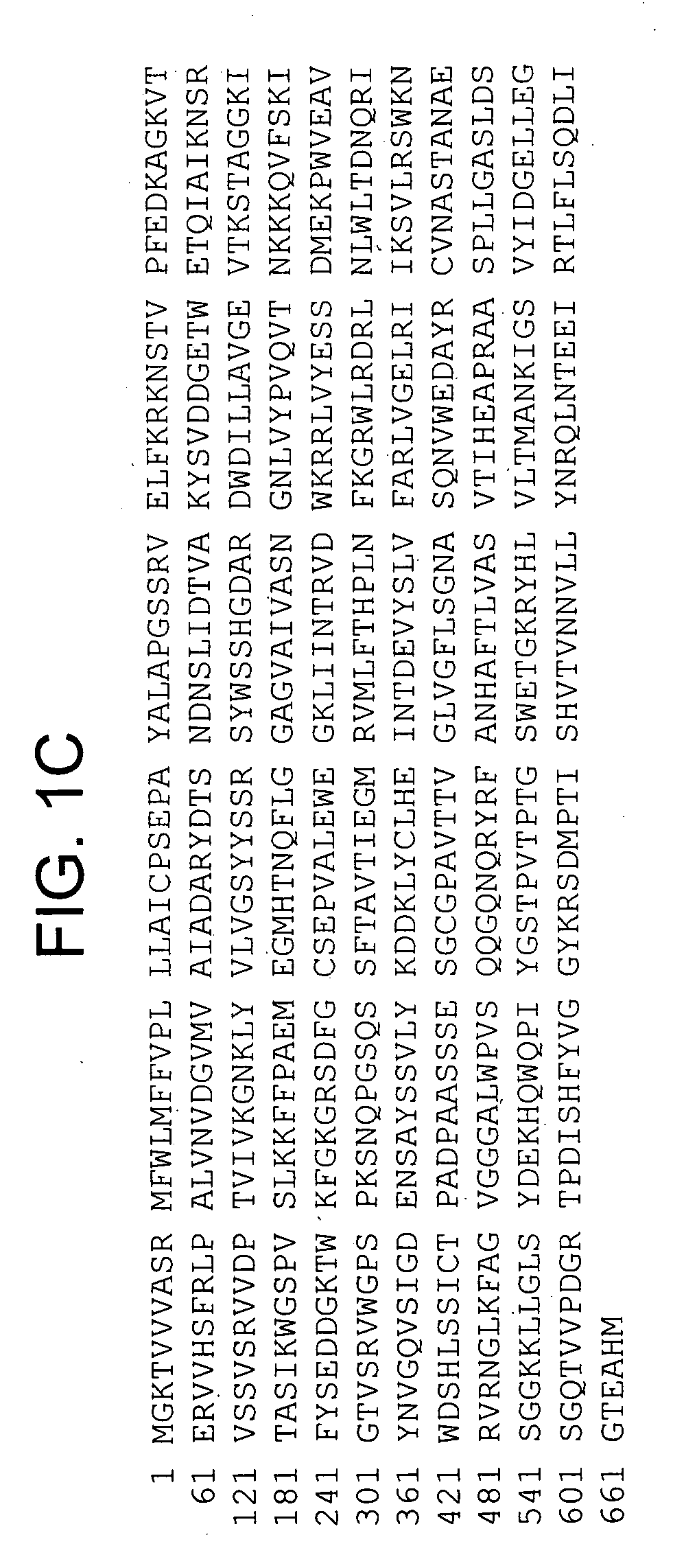
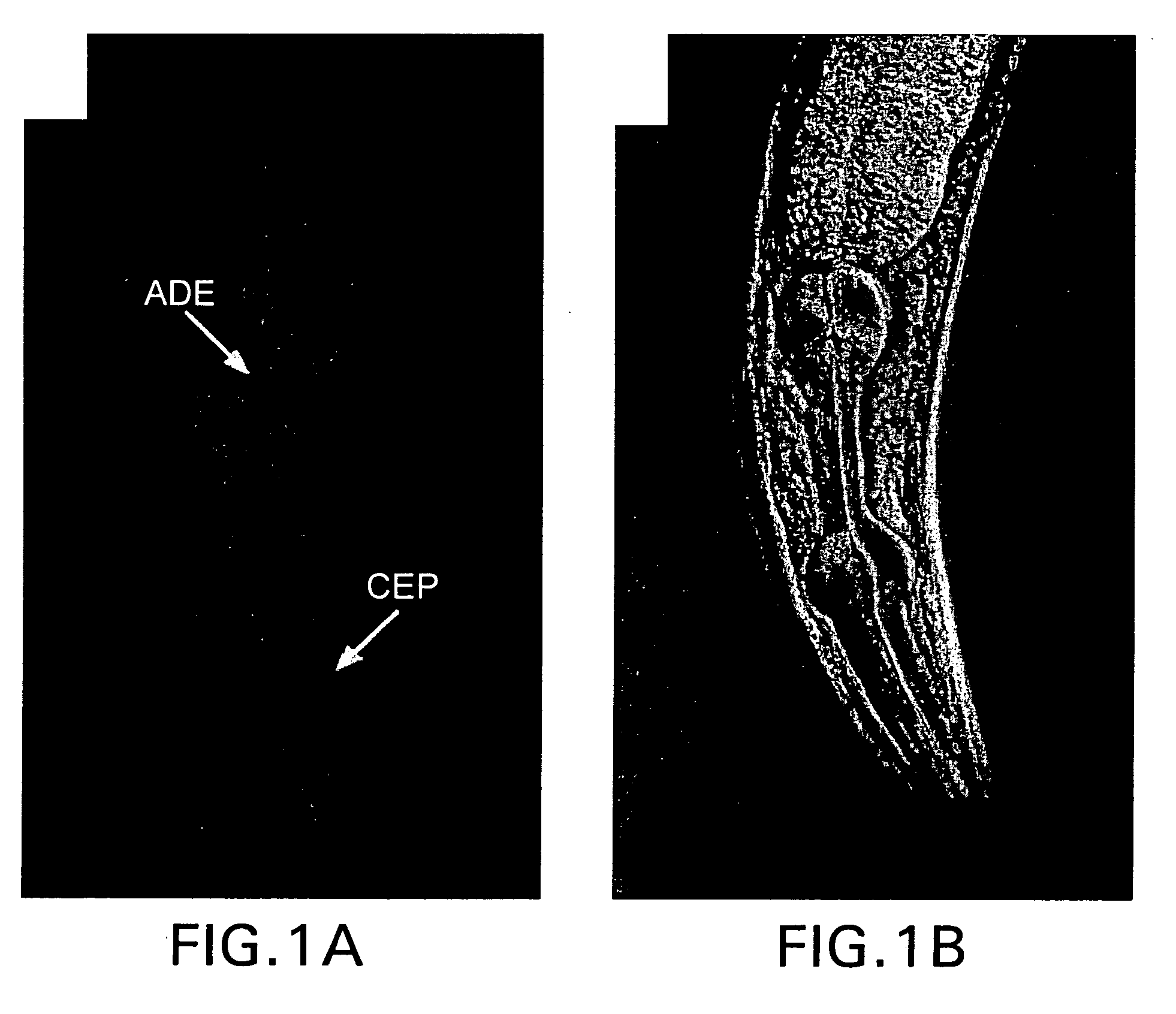
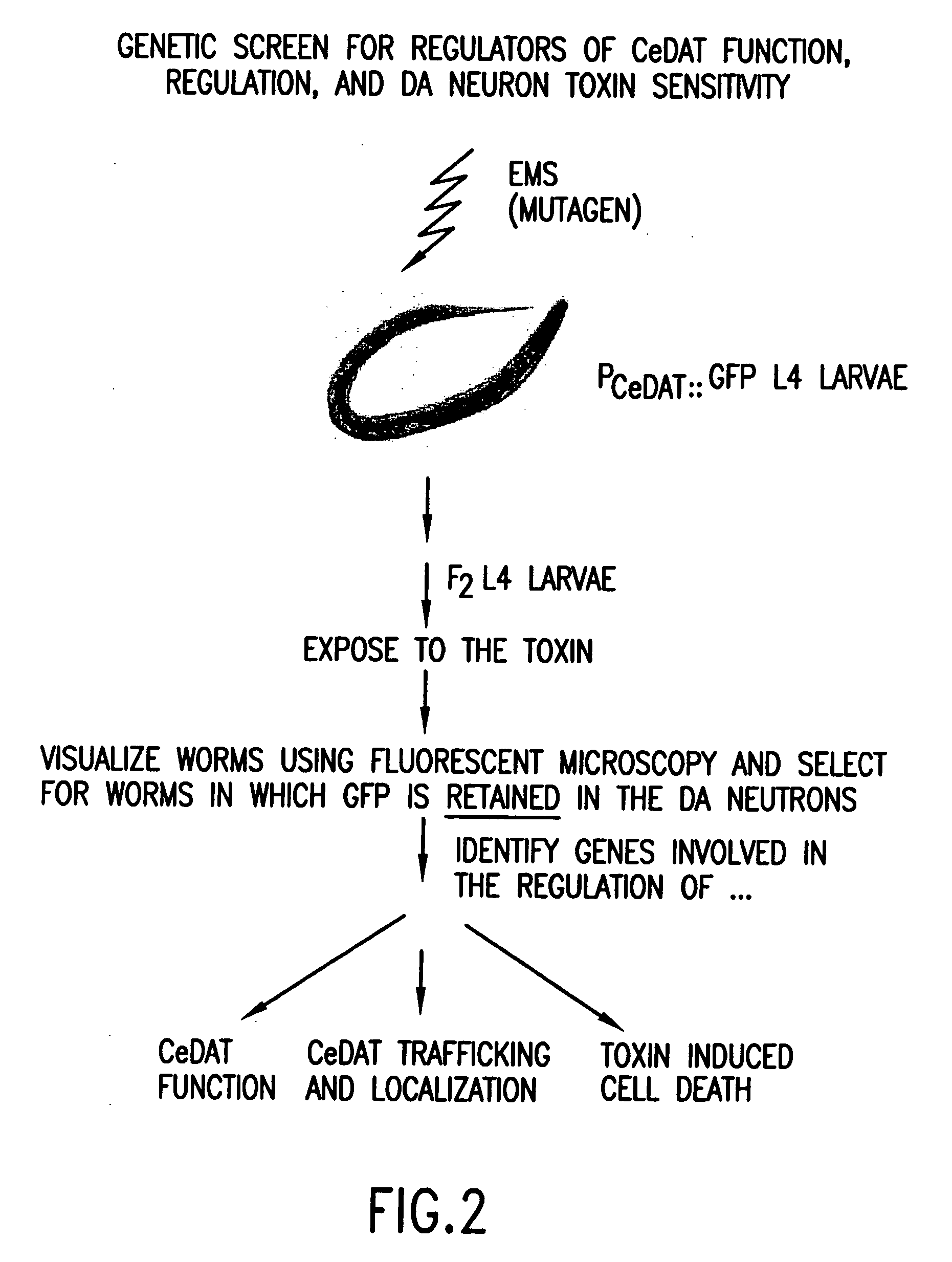
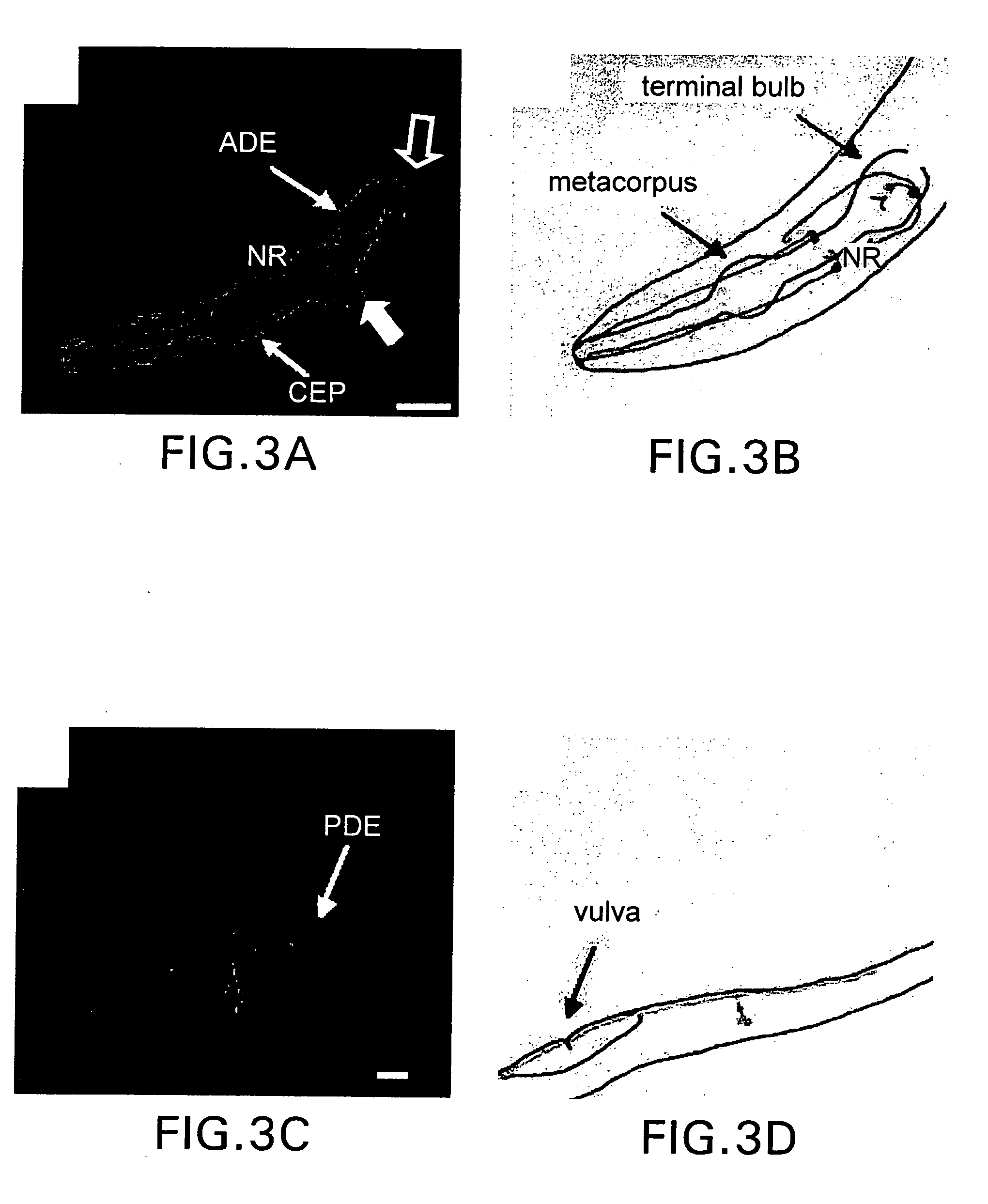
Assay for toxin induced neuronal degeneration and viability in C. elegans
InactiveUS20050160482A1Efficient screeningHigh-throughput in vivo screeningCompound screeningVectorsNeuronal degenerationNematode
Provided are in vivo screening methods to detect and identify substances that affect neuronal viability, and / or prevent neurodegeneration, and / or confer neuroprotective effects The screening methods utilize recombinant C. elegans expressing a detectable marker in neuronal sub-groups and the use of neurotoxins specific to specific neuronal cells. Also provided are methods for identifying modulators of neurotransmitter transporters such as the dopamine transporter. Therefore, the invention provides methods for identifying substances that can be used in the prevention and therapy of neurodegenerative diseases.
Owner:VANDERBILT UNIV
Methods and compositions to inhibit p2x7 receptor expression
InactiveCN101068927ACell receptors/surface-antigens/surface-determinantsGenetic material ingredientsNeuronal degenerationBiology
Methods and compositions for the downregulation of P2X7 receptor expression or activity are disclosed. Preferred compositions comprise siNA. The methods and compositions are useful in the treatment of diseases characterised by increased P2X7 receptor activity, such as neuronal degeneration, Alzheimer's disease, inflammatory diseases, and some cancers.
Owner:SYLENTIS
Methods and Compositons for Stimulating Neurogenesis and Inhibiting Neuronal Degeneration
The present invention provides methods and compositions comprising compounds useful for stimulating neurogenesis. The methods and compositions comprising compounds are also useful for inhibiting neuronal degeneration. Thus, the present invention can be used in the treatment of diseases and conditions characterized by neuronal loss and reduced neurogenesis including Alzheimer's disease, stroke, traumatic brain injury, and depression. This invention could also be used for research products including single agents or mixtures of agents to promote, proliferate, differentiate, or maintain neurons from stem or progenitor cells.
Owner:NEURONASCENT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com