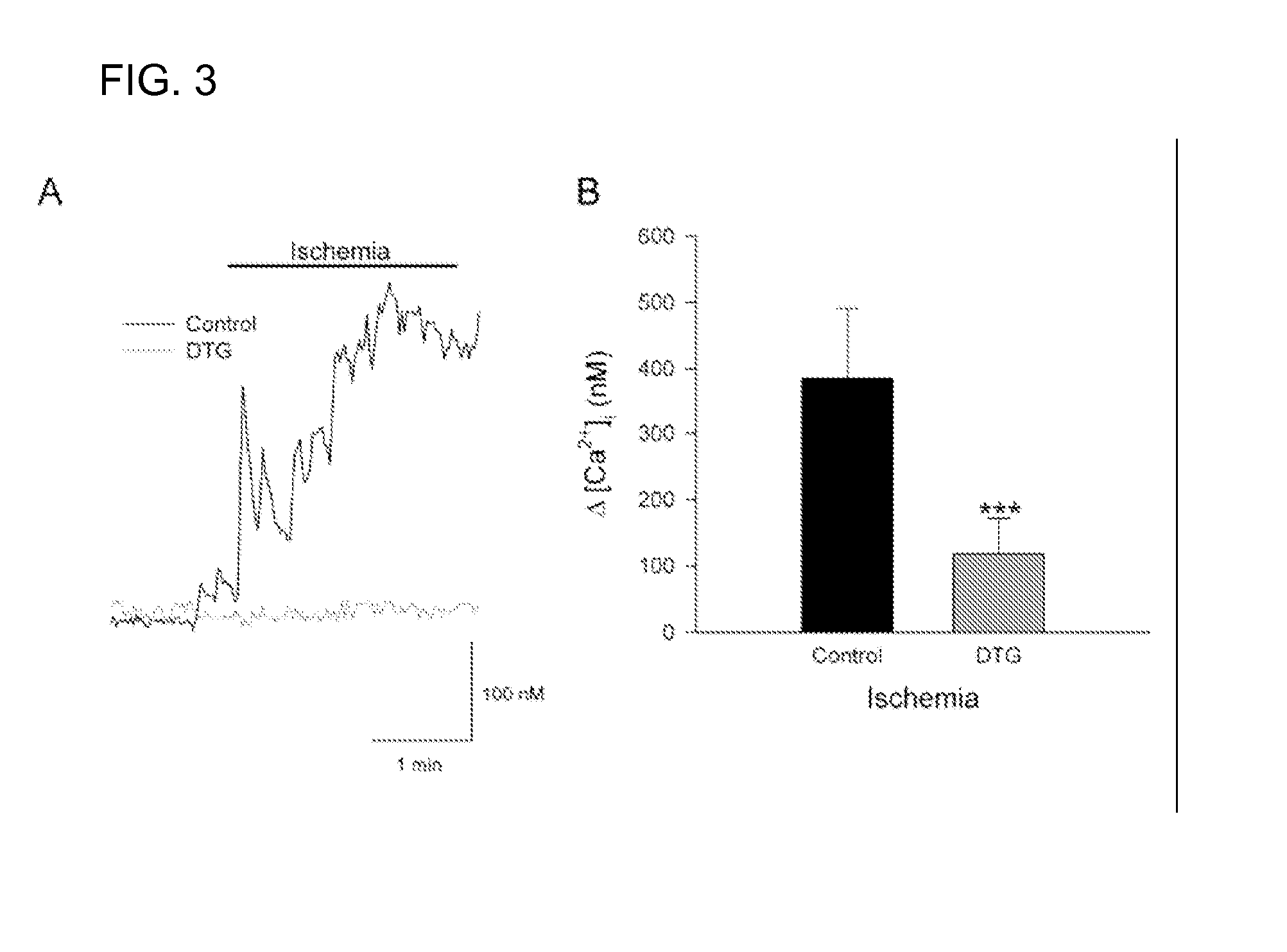Patents
Literature
74 results about "Neuron survival" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Survival of motor neuron or survival motor neuron (SMN) is a protein that in humans is encoded by the SMN1 and SMN2 genes. SMN is found in the cytoplasm of all animal cells and also in the nuclear gems.
Neural regeneration peptides and methods for their use in treatment of brain damage
ActiveUS20050131212A1High expressionEasy SurvivalPeptide/protein ingredientsGenetic material ingredientsNervous systemInjury brain
The invention discloses a family of peptides termed NRP compounds or NRPs that can promote neuronal migration, neurite outgrowth, neuronal proliferation, neural differentiation and / or neuronal survival, and provides compositions and methods for the use of NRPs in the treatment of brain injury and neurodegenerative disease. NRP compounds can induce neurons and neuroblasts to proliferate and migrate into areas of damage caused by acute brain injury or chronic neurodegenerative disease, such as exposure to toxins, stroke, trauma, nervous system infections, demyelinating diseases, dementias, and metabolic disorders. NRP compounds may be administered directly to a subject or to a subject's cells by a variety of means including orally, intraperitoneally, intravascularly, and directly into the nervous system of a patient. NRP compounds can be formulated into pharmaceutically acceptable dose forms for therapeutic use. Methods for detecting neural regeneration, neural proliferation, neural differentiation, neurite outgrowth and neural survival can be used to develop other neurally active agents.
Owner:CURONZ HLDG
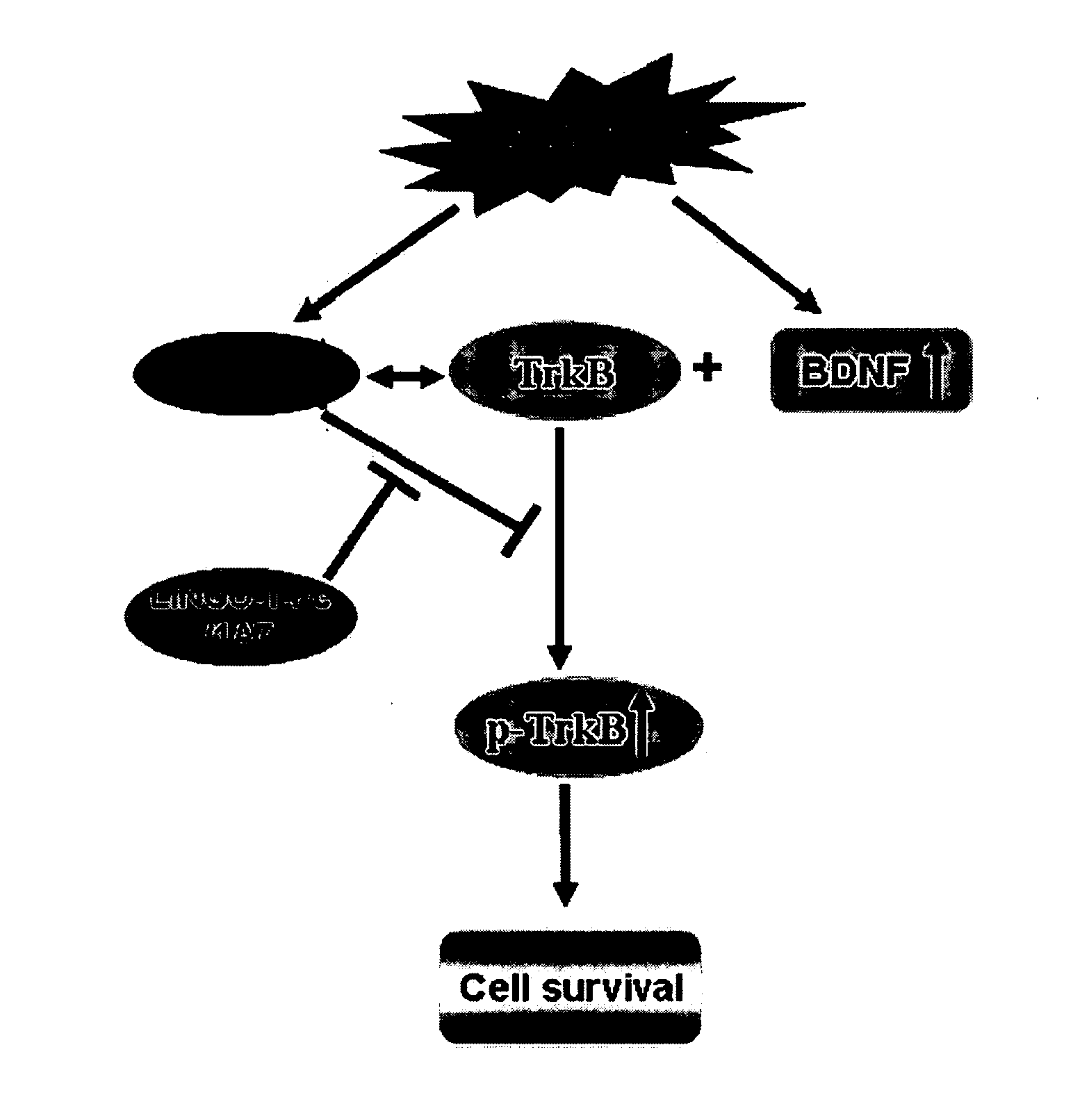
Methods for Treating Pressure Induced Optic Neuropathy, Preventing Neuronal Degeneration and Promoting Neuronal Cell Survival Via Administration of LINGO-1 Antagonists and TrkB Agonists
InactiveUS20100297121A1High activityPromote cell activationOrganic active ingredientsSenses disorderNeuronal degenerationMedicine
This invention relates to methods for promoting neuronal survival and regeneration using LINGO-1 antagonists and TrkB agonists. Additionally, the invention relates to methods for treating pressure induced optic neuropathies using LINGO-1 antagonists. The invention also relates generally to methods for increasing TrkB activity and inhibiting JNK pathway signaling using a LINGO-1 antagonist.
Owner:BIOGEN MA INC
System and method of contra-lateral ear stimulation for preserving neuronal survival and plasticity of the auditory system prior to permanent intra-cochlear implantation
InactiveUS20070282396A1Preserving neuronal survivalPreserving plasticityElectrotherapyArtificial respirationAuditory pathwaysAuditory system
An implantable microstimulator, or equivalent neural stimulator, generates a relatively simple signal containing temporally challenging information and delivers such signal to the middle or outer portion of the ear contra-lateral to an ear having a cochlear implant of a bilaterally deafened patient. Such stimulation delivered to the contra lateral ear advantageously increases the survival rate of neurons therein, and further helps maintain or extend the plasticity of the higher auditory pathways of the contra-lateral ear, thereby allowing a cochlear implant to be more effectively used in such ear at a later date. The stimulation provided by the microstimulator, or equivalent simple neural stimulator, need not be continuous, but may be provided only during limited periods of time each day, or only on selected days. Further, such stimulation preserves whatever residual hearing may be left in the contra lateral ear.
Owner:BOSTON SCI NEUROMODULATION CORP
Treatment with Sigma Receptor Agonists Post-Stroke
InactiveUS20070123556A1Decrease in infarction areaHigh affinityBiocidePeptide/protein ingredientsHippocampal regionGlial fibrillary acidic protein
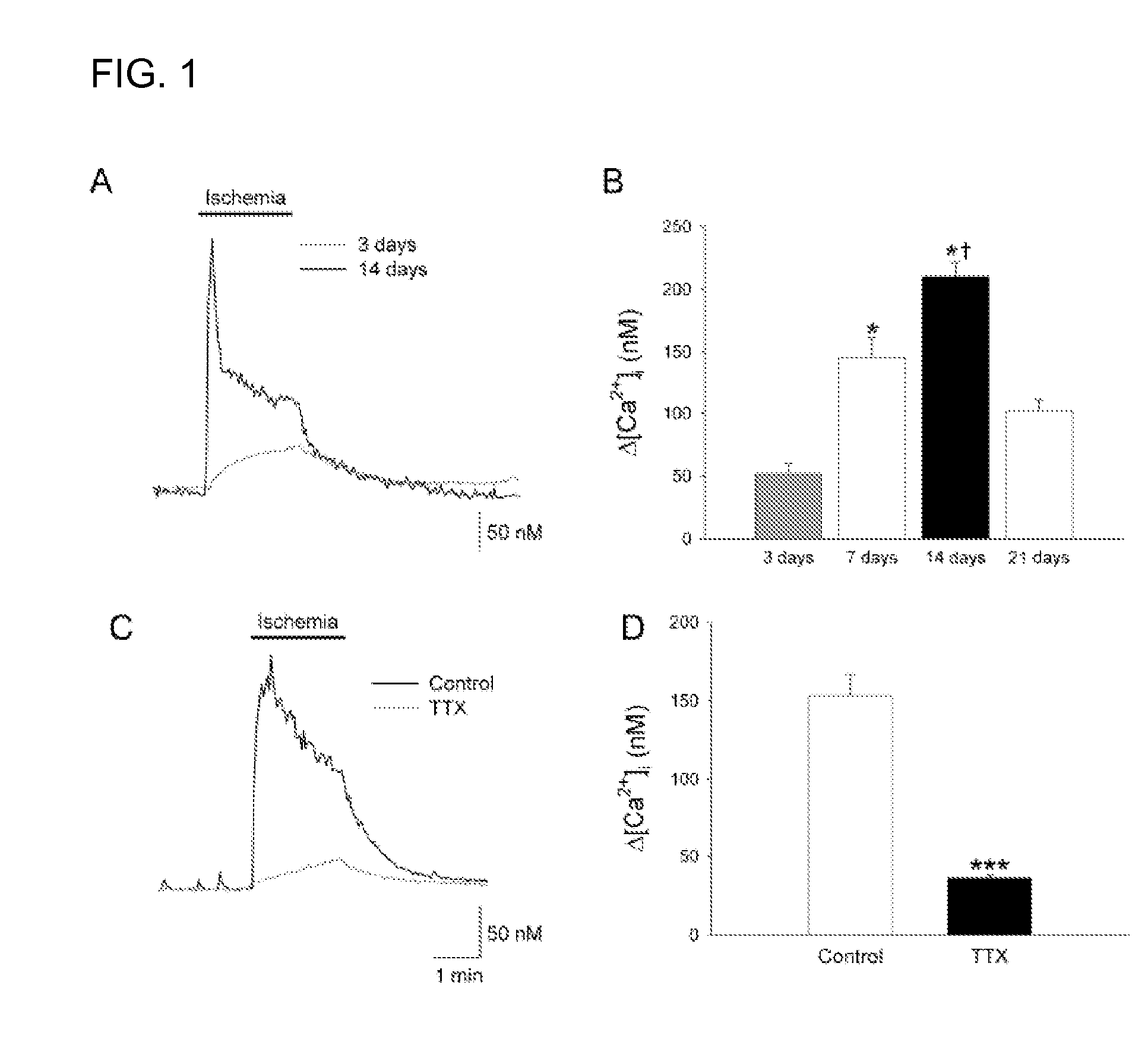
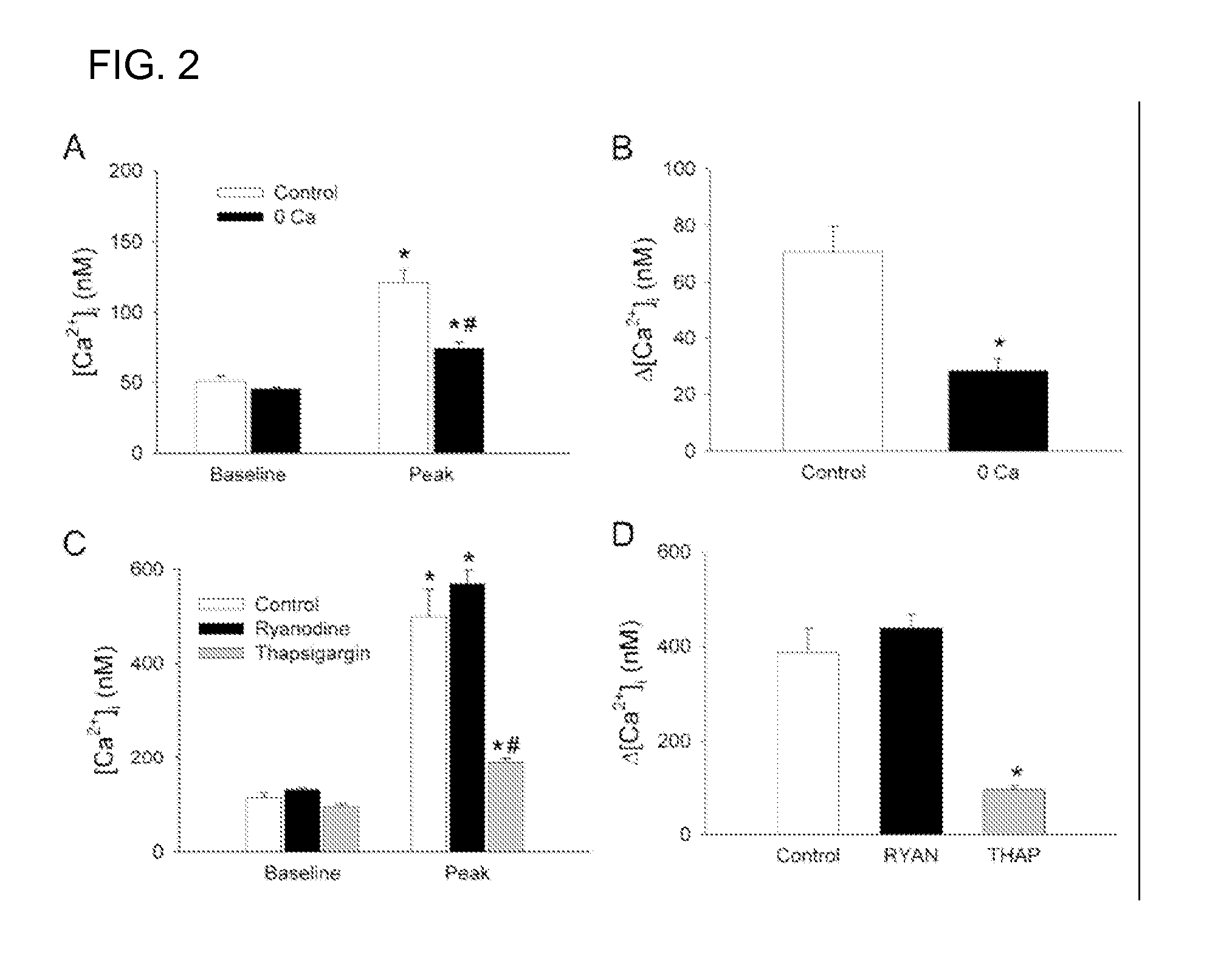
A method of post-stroke treatment at delayed timepoints with sigma receptor agonists. Sigma receptors are promising targets for neuroprotection following ischemia. One of the key components in the demise of neurons following ischemic injury is the disruption of intracellular calcium homeostasis. The sigma receptor agonist, DTG, was shown to depress [Ca2+]i elevations observed in response to ischemia induced by sodium azide and glucose deprivation. Two sigma receptor antagonists, metaphit and BD-1047, were shown to blunt the ability of DTG to inhibit ischemia-evoked increases in [Ca2+]i. DTG inhibition of ischemia-induced increases in [Ca2+]i was mimicked by the sigma-1 receptor-selective agonists, carbetapentane, (+)-pentazocine and PRE-084, but not by the sigma-2 selective agonist, ibogaine, showing that activation of sigma-1 receptors is responsible for the effects. Activation of sigma receptors can ameliorate [Ca2+]i dysregulation associated with ischemia in cortical neurons, providing neuroprotective properties. The effects of 1,3-di-o-tolyguanidine (DTG), a high affinity sigma receptor agonist, as a potential treatment for decreasing infarct area at delayed time points was further examined in rats. DTG treatment significantly reduced infarct area in both cortical / striatal and cortical / hippocampal regions by >80%, relative to control rats. These findings were confirmed by immunohistochemical experiments using the neuronal marker, mouse anti-neuronal nuclei monoclonal antibody (NeuN), which showed that application of DTG significantly increased the number of viable neurons in these regions. Furthermore, DTG blocked the inflammatory response evoked by MCAO, as indicated by decreases in the number of reactive astrocytes and activated microglia / macrophages detected by immunostaining for glial fibrillary acidic protein (GFAP) and binding of isolectin IB4, respectively. Thus, the sigma receptor-selective agonist, DTG, can enhance neuronal survival when administered 24 hr after an ischemic stroke. In addition, the efficacy of sigma receptors for stroke treatment at delayed time points is likely the result of combined neuroprotective and anti-inflammatory properties of these receptors.
Owner:UNIV OF SOUTH FLORIDA
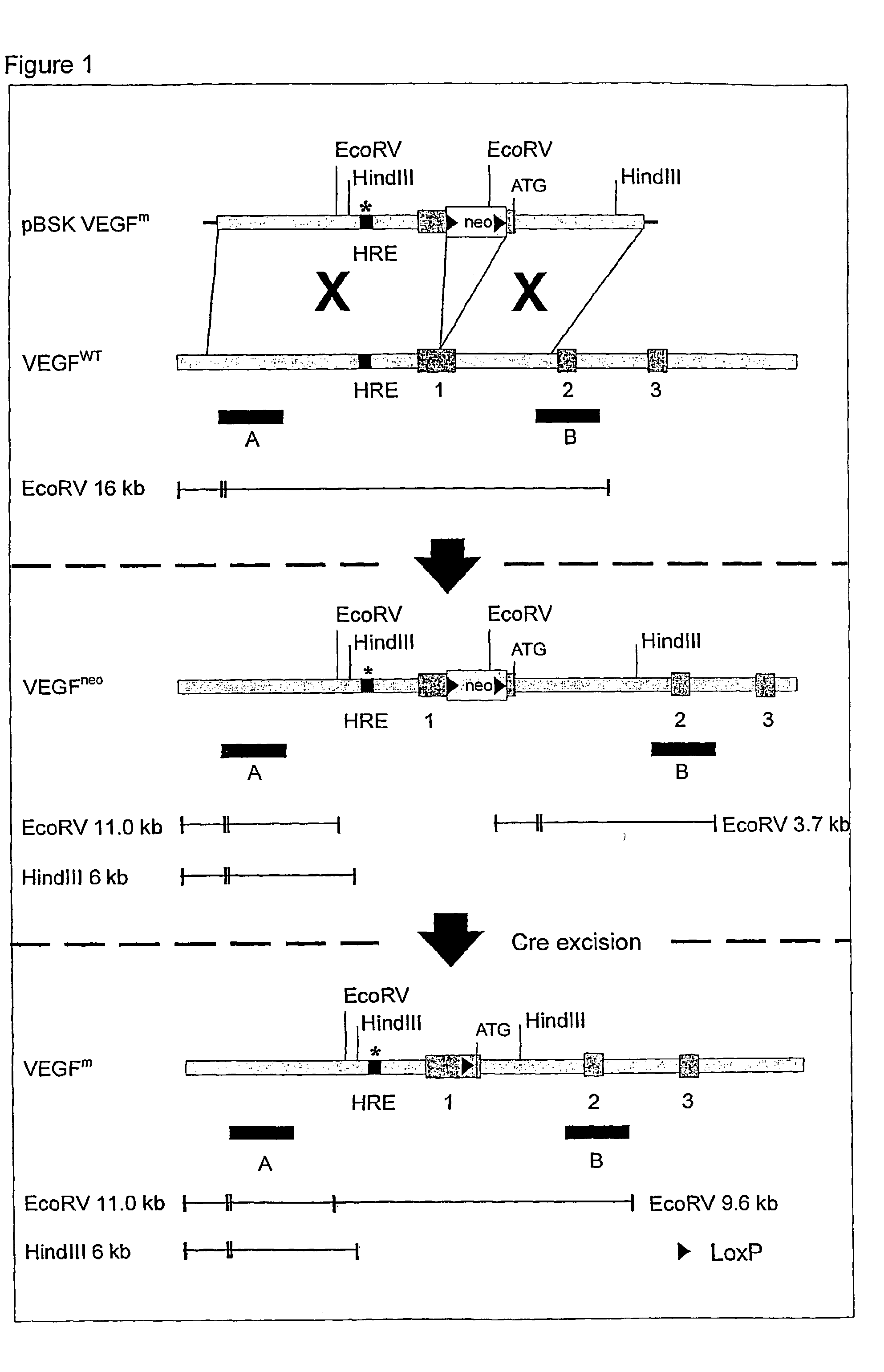
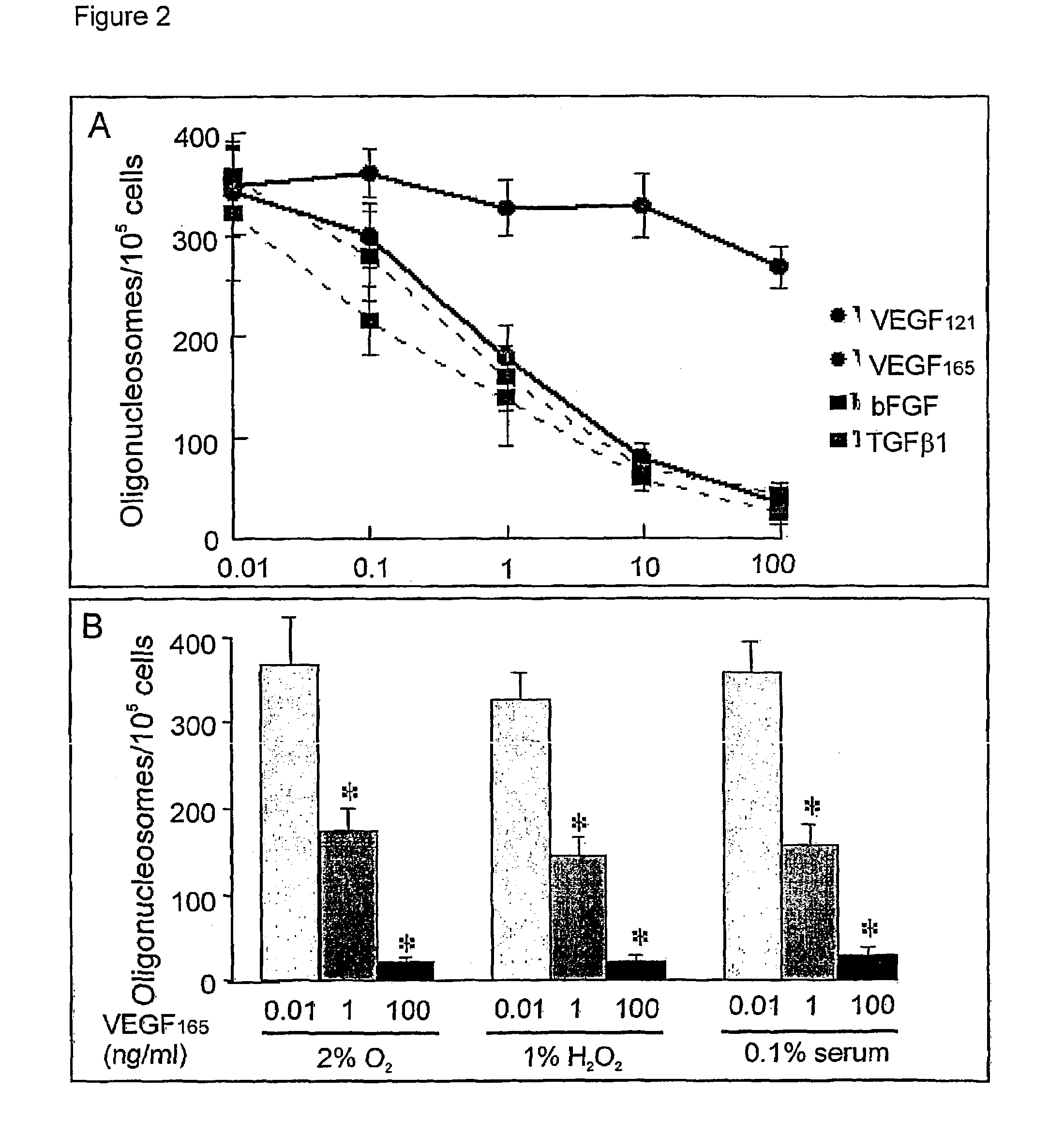
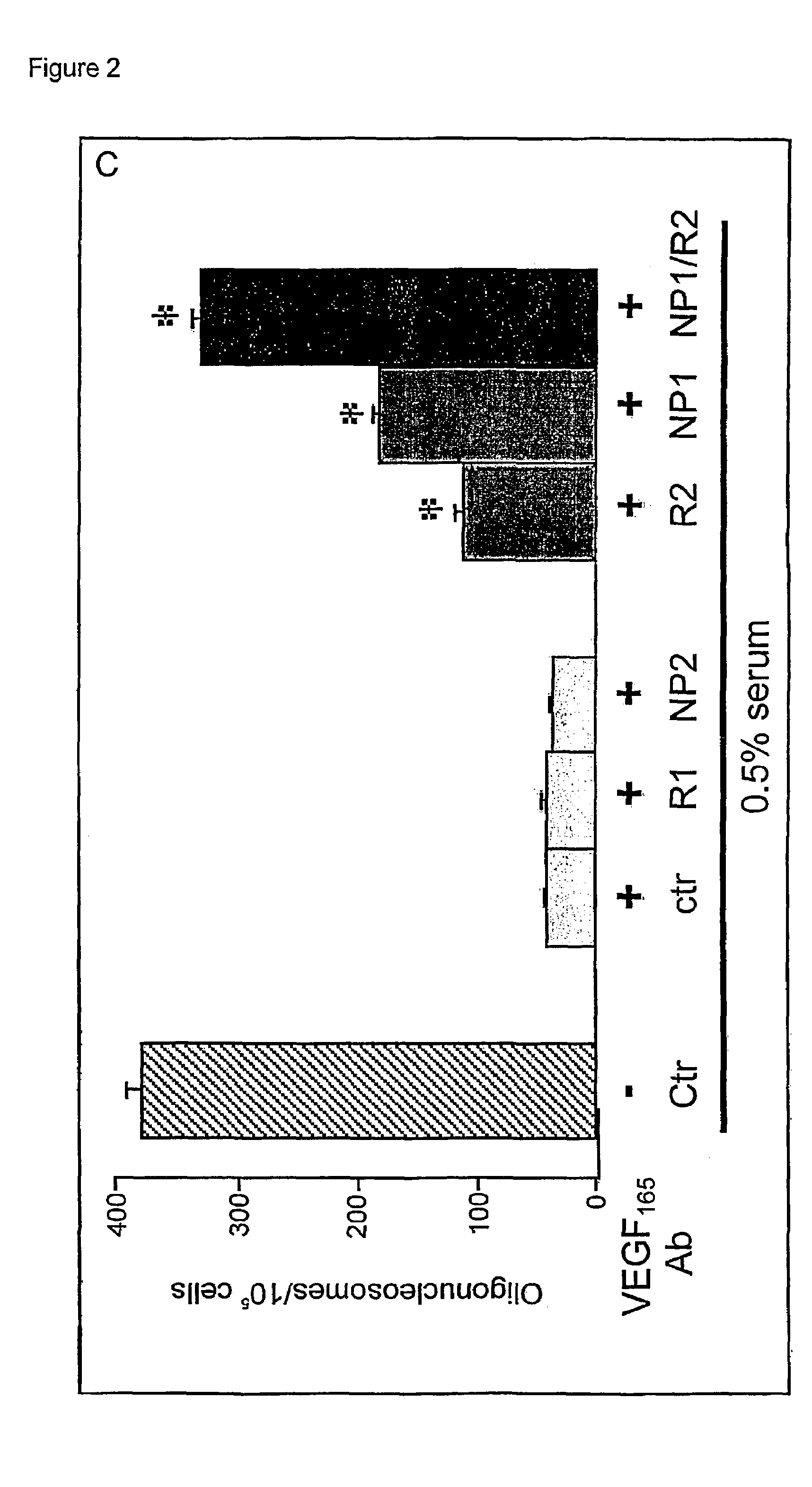
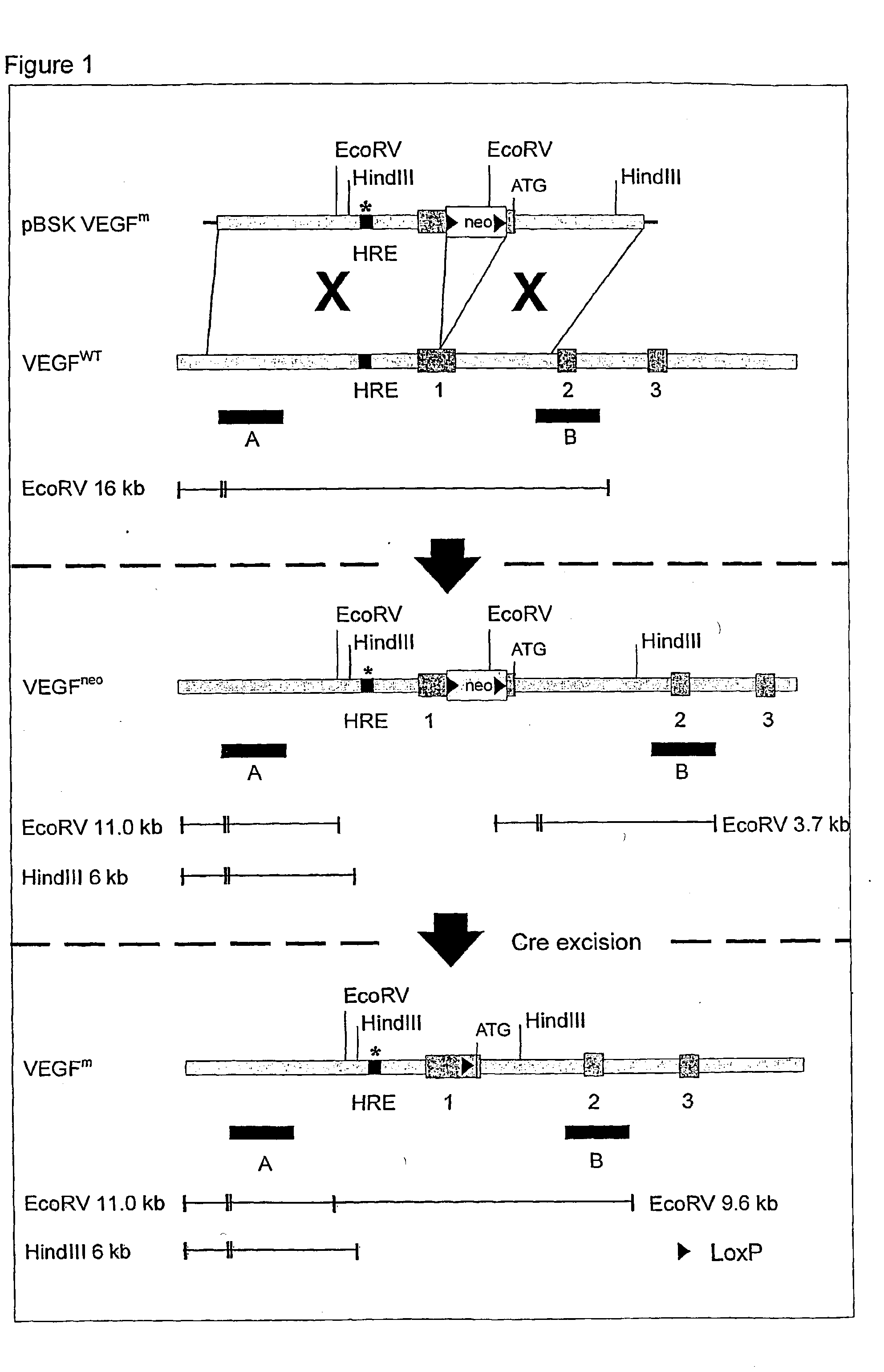
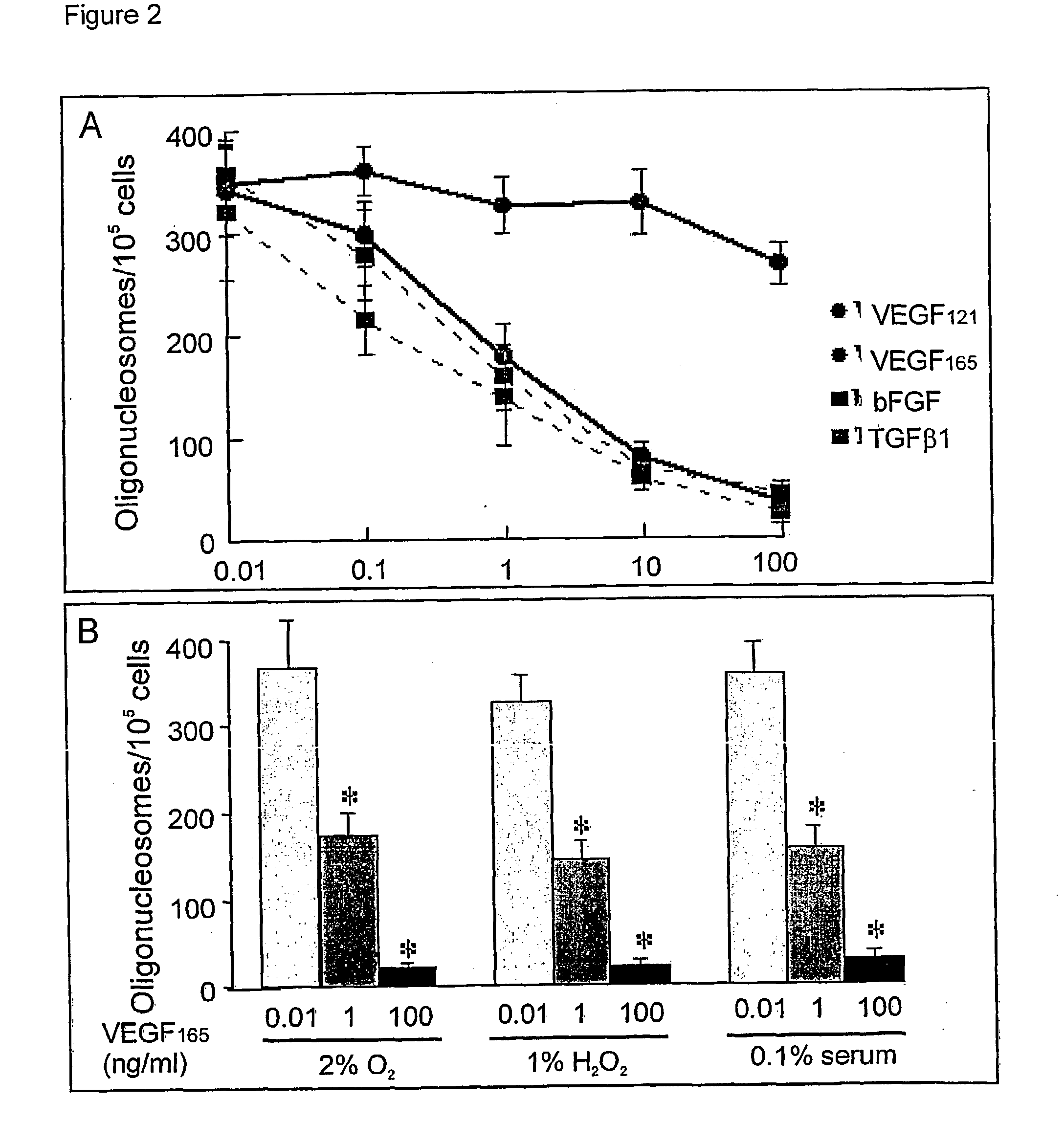
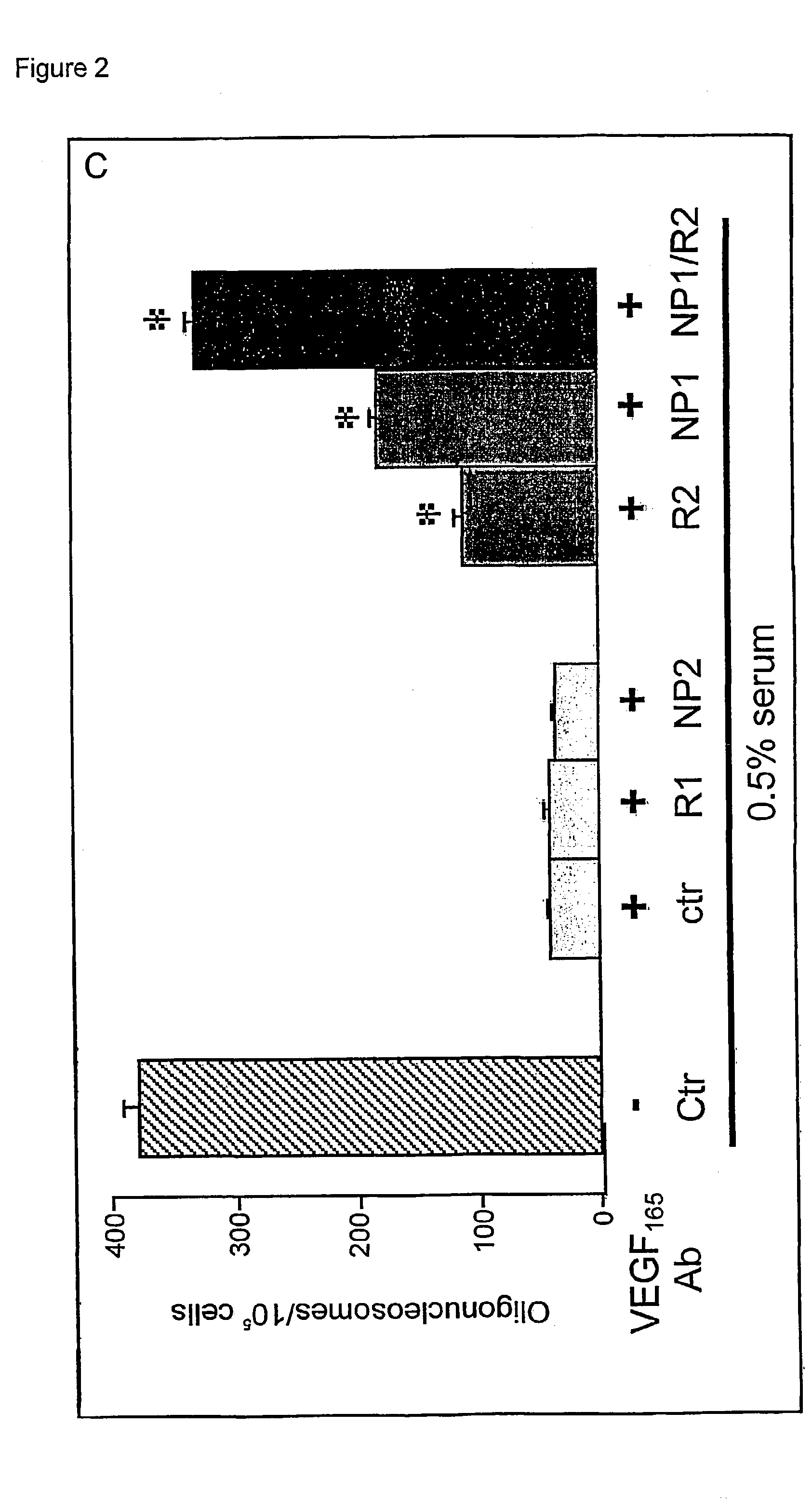
Use of VEGF and homologues to treat neuron disorders
InactiveUS7226908B2Nervous disorderPeptide/protein ingredientsTruncal muscle weaknessSurvival of motor neuron
The present invention relates to neurological and physiological dysfunction associated with neuron disorders. In (particular, the invention relates to the involvement of vascular endothelial growth factor (VEGF) and homologues in the aetiology of motor neuron disorders. The invention further concerns a novel, mutant transgenic mouse (VEGFm / m) with a homozygous deletion in the hypoxia responsive element (HRE) of the VEGF promoter which alters the hypoxic upregulation of VEGF. These mice suffer severe adult onset muscle weakness due to progressive spinal motor neuron degeneration which is reminiscent of amyotrophic lateral sclerosis (ALS)—a fatal disorder with unknown aetiology. Furthermore, the neuropathy of these mice is not caused by vascular defects, but is due to defective VEGF-mediated survival signals to motor neurons. The present invention relates in particular to the isoform VEGF165 which stimulates survival of motor neurons via binding to neuropilin-1, a receptor known to bind semaphorin-3A which is implicated in axon retraction and neuronal death, and the VEGF Receptor-2. The present invention thus relates to the usage of VEGF, in particular VEGF165, for the treatment of neuron disorders and relates, in addition, to the usage of polymorphisms in the VEGF promotor for diagnosing the latter disorders.
Owner:LIFE SCI RES PARTNERS VZW +1
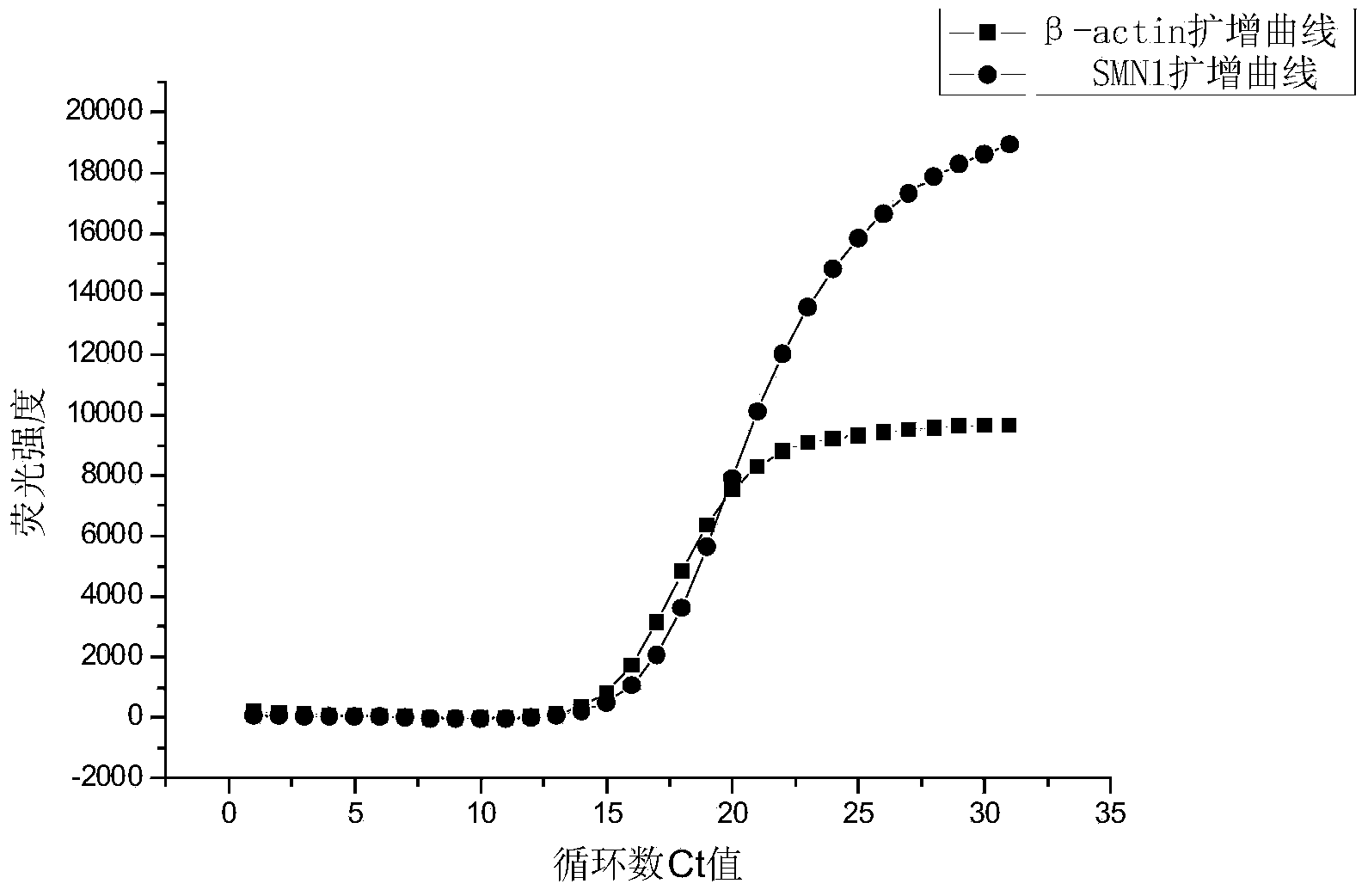
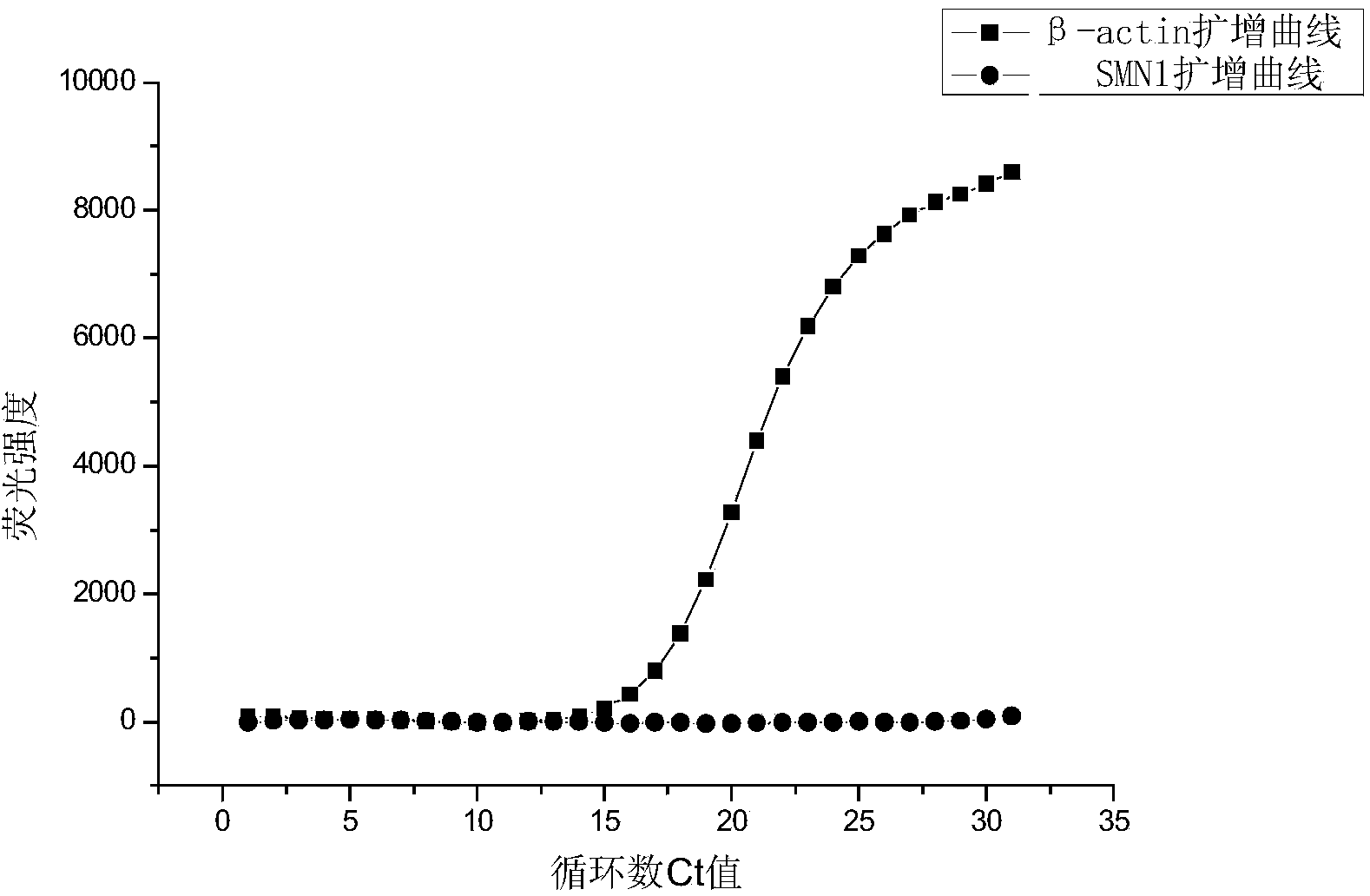
Primer and probe for screening spinal muscular atrophy (SMA) genes and using method of primer and probe
InactiveCN103555835ADifferentiate severityMicrobiological testing/measurementDNA/RNA fragmentationGenotypePrimary motor neuron
The invention discloses a primer and a probe for screening spinal muscular atrophy (SMA) genes and a using method of the primer and probe, belonging to the technical field of biology. The invention discloses eight combinations of the primer and probe for screening survival motor neuron genes 1 (SMN1), and the primer and probe can be effectively applied to screening the SMN1. Meanwhile, the invention also discloses sixteen groups of combinations of the primer and probe for screening survival motor neuron genes 2 (SMN2), as well as application in fetal SMA gene screening by utilizing the primer and probe for screening the SMN1 and the primer and probe for screening the SMN2. According to the primer and probe provided by the invention, the SMA genotypes of adults and fetuses can be detected at high efficiency.
Owner:曾骥孟
Use of vegf and homologues to treat neuron disorders
InactiveUS20030105018A1Impaired hypoxic upregulationDeterioration progressNervous disorderPeptide/protein ingredientsTruncal muscle weaknessSurvival of motor neuron
The present invention relates to neurological and physiological dysfunction associated with neuron disorders. In (particular, the invention relates to the involvement of vascular endothelial growth factor (VEGF) and homologues in the aetiology of motor neuron disorders. The invention further concerns a novel, mutant transgenic mouse (VEGFm / m) with a homozygous deletion in the hypoxia responsive element (HRE) of the VEGF promoter which alters the hypoxic upregulation of VEGF. These mice suffer severe adult onset muscle weakness due to progressive spinal motor neuron degeneration which is reminiscent of amyotrophic lateral sclerosis (ALS)-a fatal disorder with unknown aetiology. Furthermore, the neuropathy of these mice is not caused by vascular defects, but is due to defective VEGF-mediated survival signals to motor neurons. The present invention relates in particular to the isoform VEGF165 which stimulates survival of motor neurons via binding to neuropilin-1, a receptor known to bind semaphorin-3A which is implicated in axon retraction and neuronal death, and the VEGF Receptor-2. The present invention thus relates to the usage of VEGF, in particular VEGF165, for the treatment of neuron disorders and relates, in addition, to the usage of polymorphisms in the VEGF promotor for diagnosing the latter disorders.
Owner:LIFE SCI RES PARTNERS VZW +1
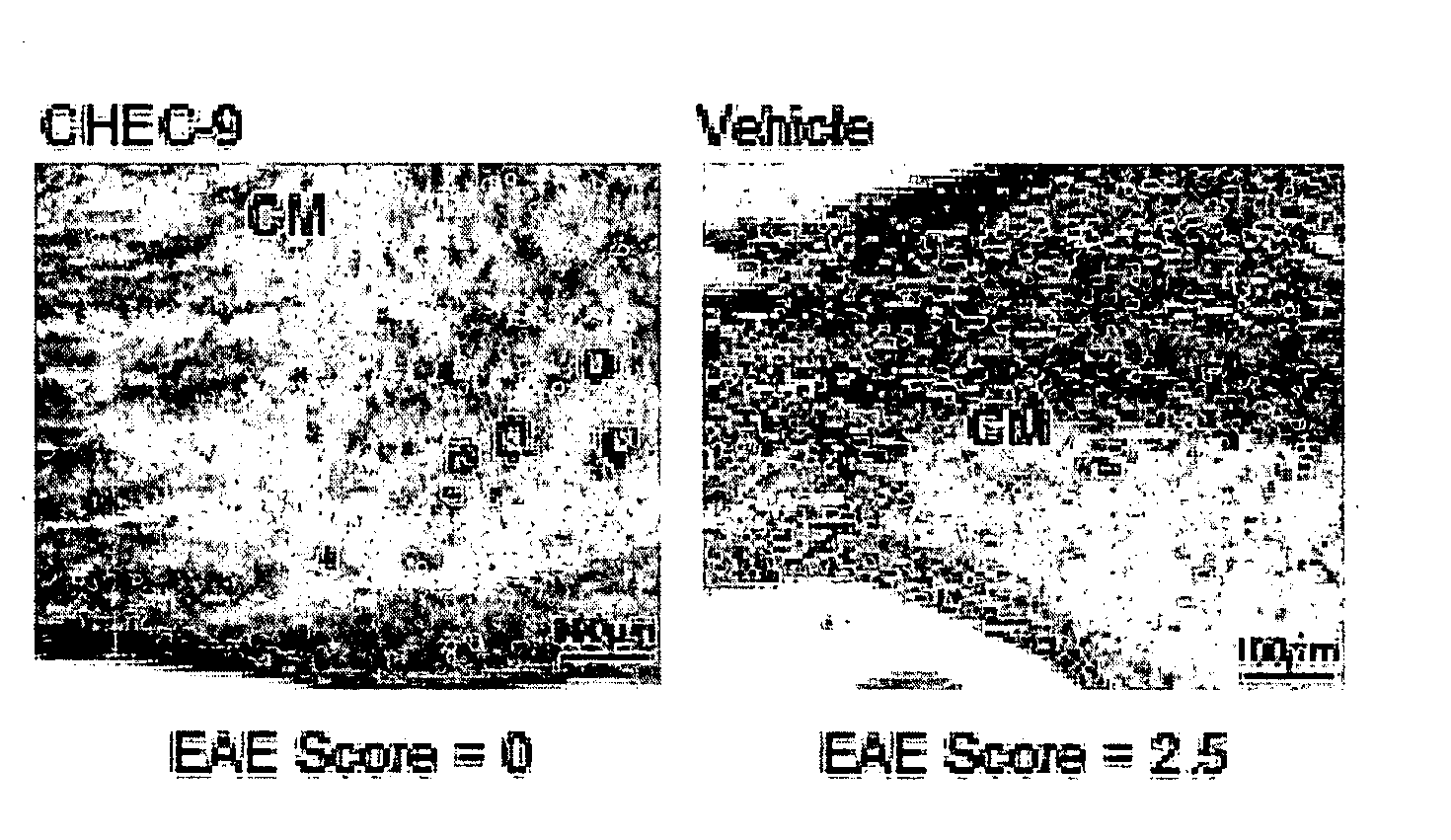
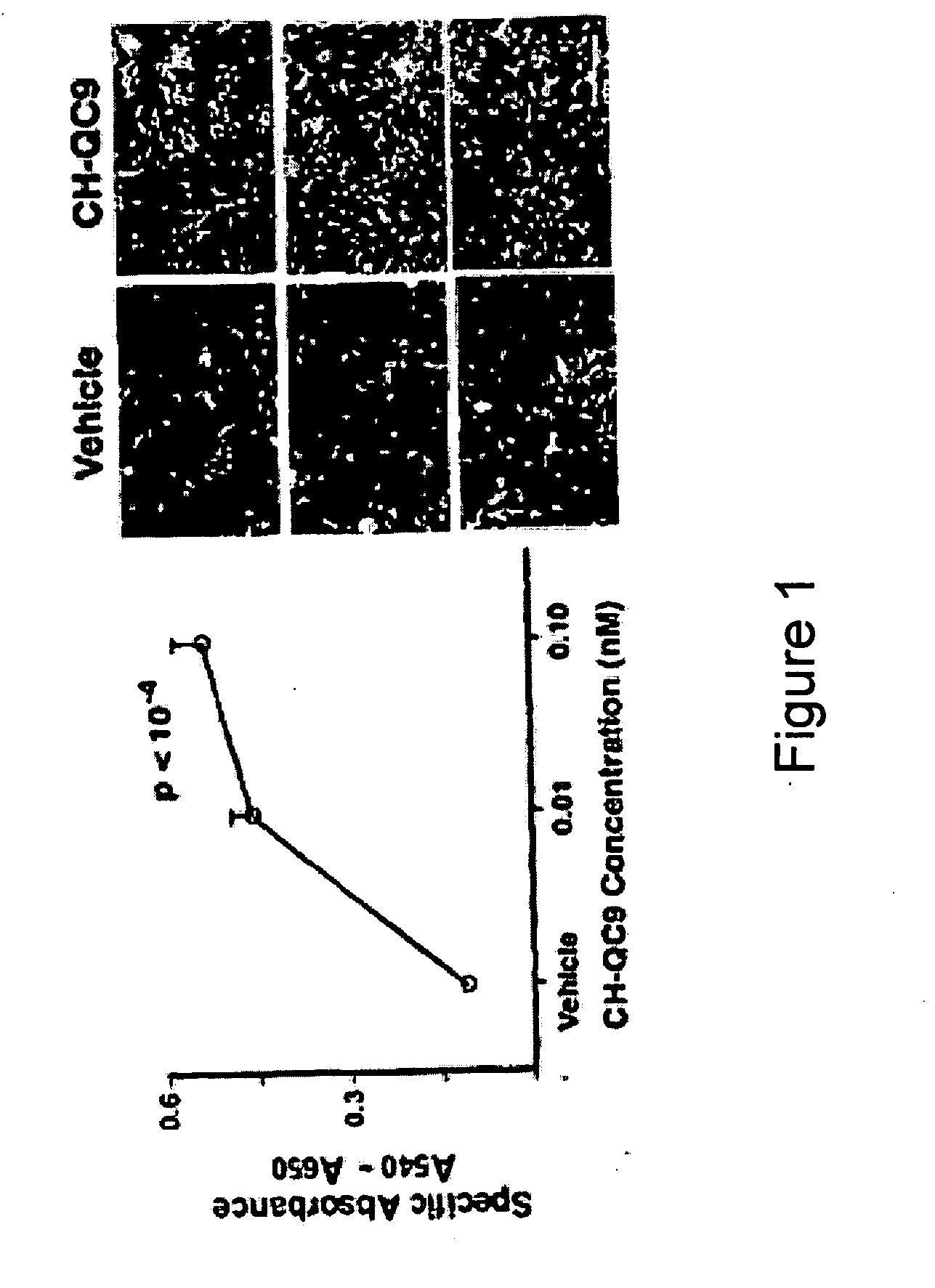
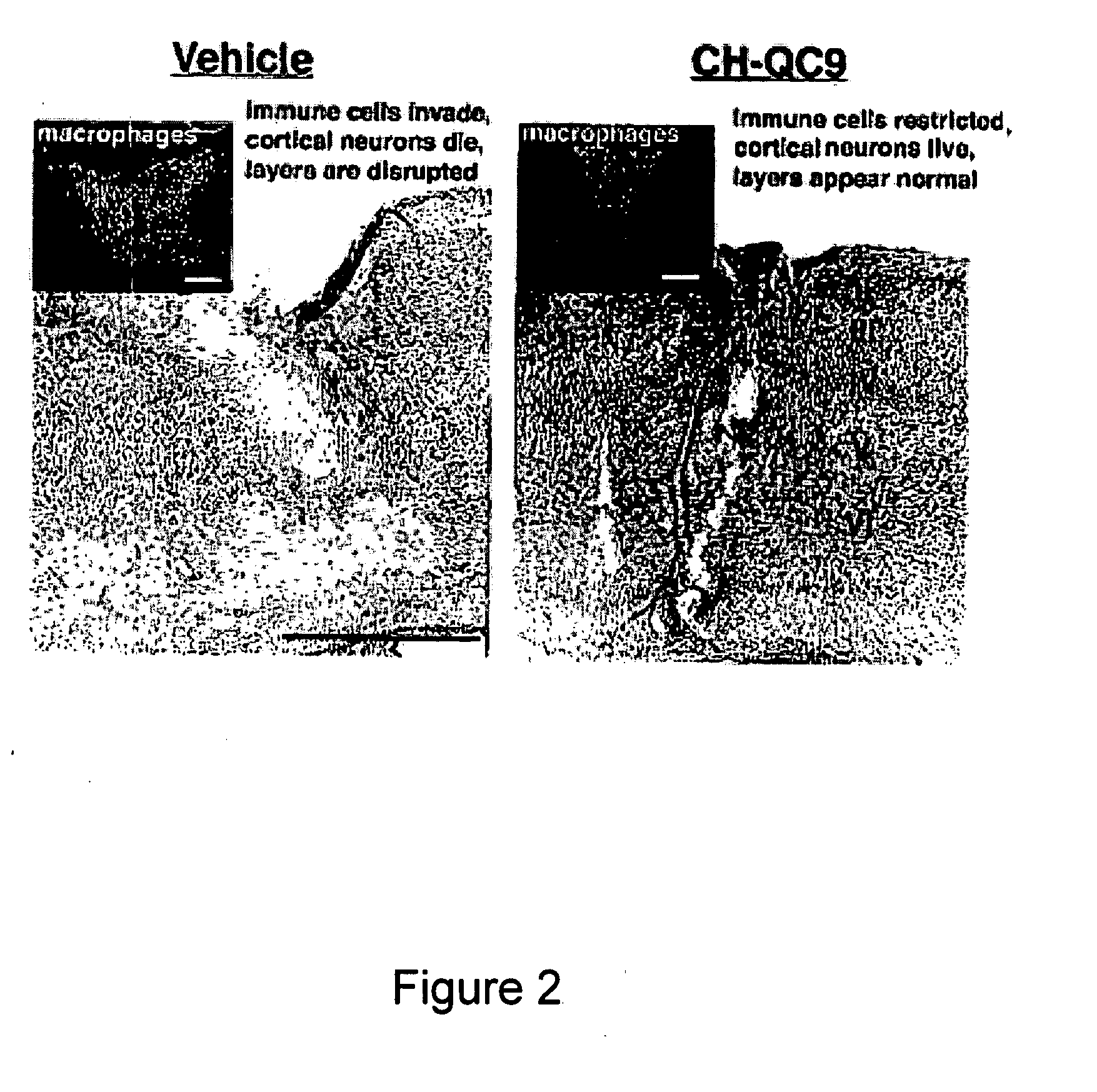
CHEC-7 a novel sPLA2 inhibitor and methods of use for treating neurological and inflammatory disorders
The present invention relates to the discovery of a composition including a seven-amino acid peptide that promotes neuronal survival, inhibits inflammation, and is a potent inhibitor of sPL2A, and uses thereof.
Owner:PHILADELPHIA HEALTH & EDUCATION CORP
System and method for preserving neuronal survival and plasticity of the auditory system prior to permanent intra-cochlear implantation
InactiveUS20070282395A1Simple signalImprove survival rateElectrotherapyArtificial respirationTotal DeafnessOlder child
A system and method uses an electrical stimulator to stimulate the auditory system with a relatively simple signal that contains temporally challenging information in order to preserve neuronal survival and plasticity of the auditory system, and also to preserve residual hearing. The stimulation provided need not be continuous, but may be provided only during limited periods of time each day, or only on selected days. The system or method is particularly suited for very young children who acquire hearing impairment or deafness early in life and who may not yet be ready for a cochlear implant. The invention requires only minimal surgical intervention, if any, and may be carried out without the need for intra-cochlear electrodes. Under special circumstances, the invention may also be used with older children or adults with a hearing impairment or deafness.
Owner:BOSTON SCI NEUROMODULATION CORP
Methods for identifying candidates for the treatment of neurodegenerative diseases
InactiveUS9733237B2Accelerating degenerationGood effectAnimal cellsOrganic active ingredientsWild typeNeuro-degenerative disease
The present invention provides, inter alia, methods for identifying a candidate agent that may be effective to treat or ameliorate an effect of a neurodegenerative disease in a subject. These methods include: (a) contacting a wildtype neuron and a mutant neuron with a stressor which is effective to accelerate the degeneration of the mutant neuron; (b) further contacting the wildtype neuron and the mutant neuron from step (a) with a candidate agent; and (c) determining whether the candidate agent lowers a wildtype to mutant survival ratio or increases both wildtype and mutant neuron survival.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Compositions, methods, and kits useful for the diagnosis and treatment of spinal muscular atrophy
InactiveUS20050100923A1Easy splicingImprove the level ofNanotechBacteriaSurvival of motor neuronSmn gene
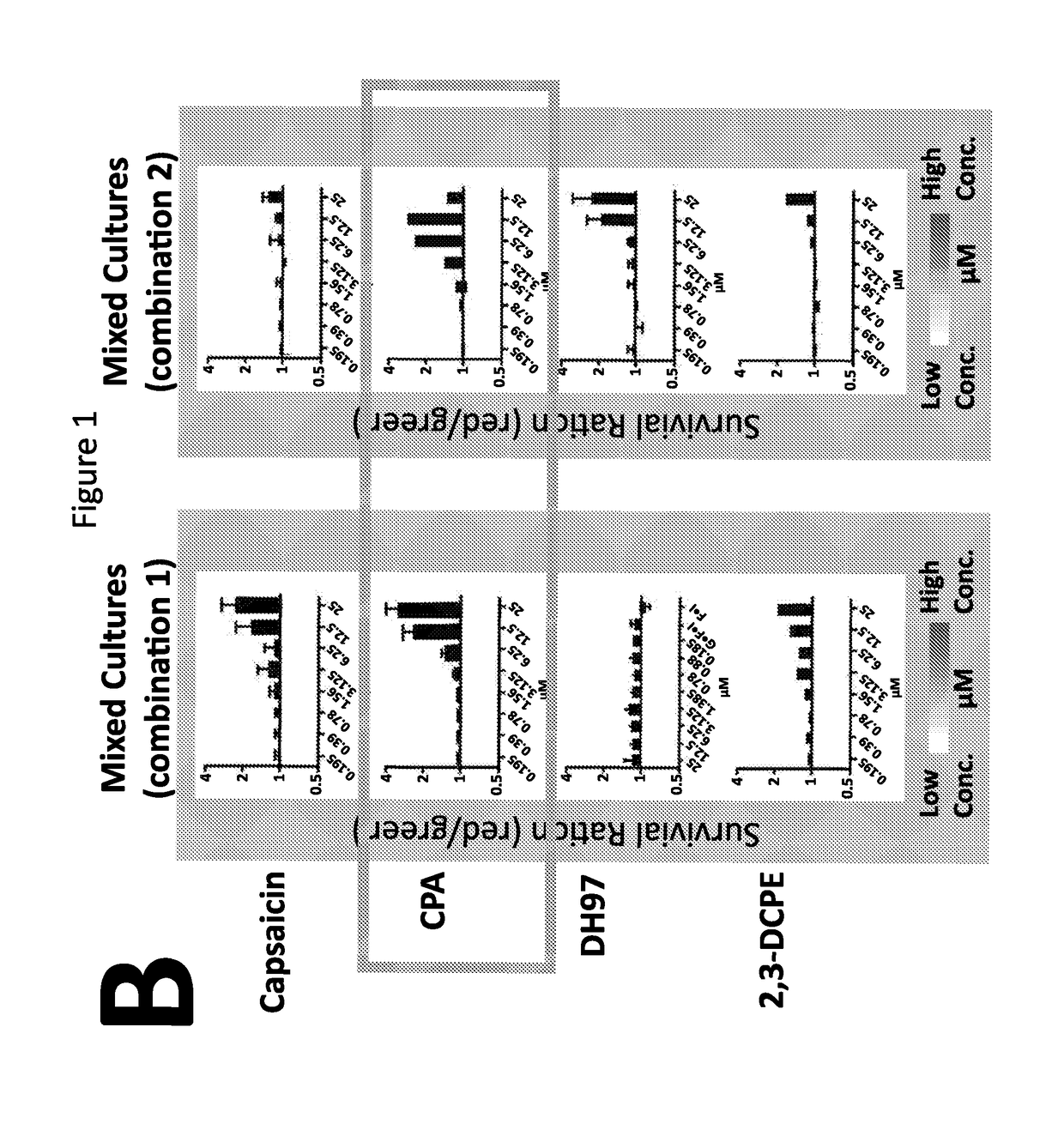
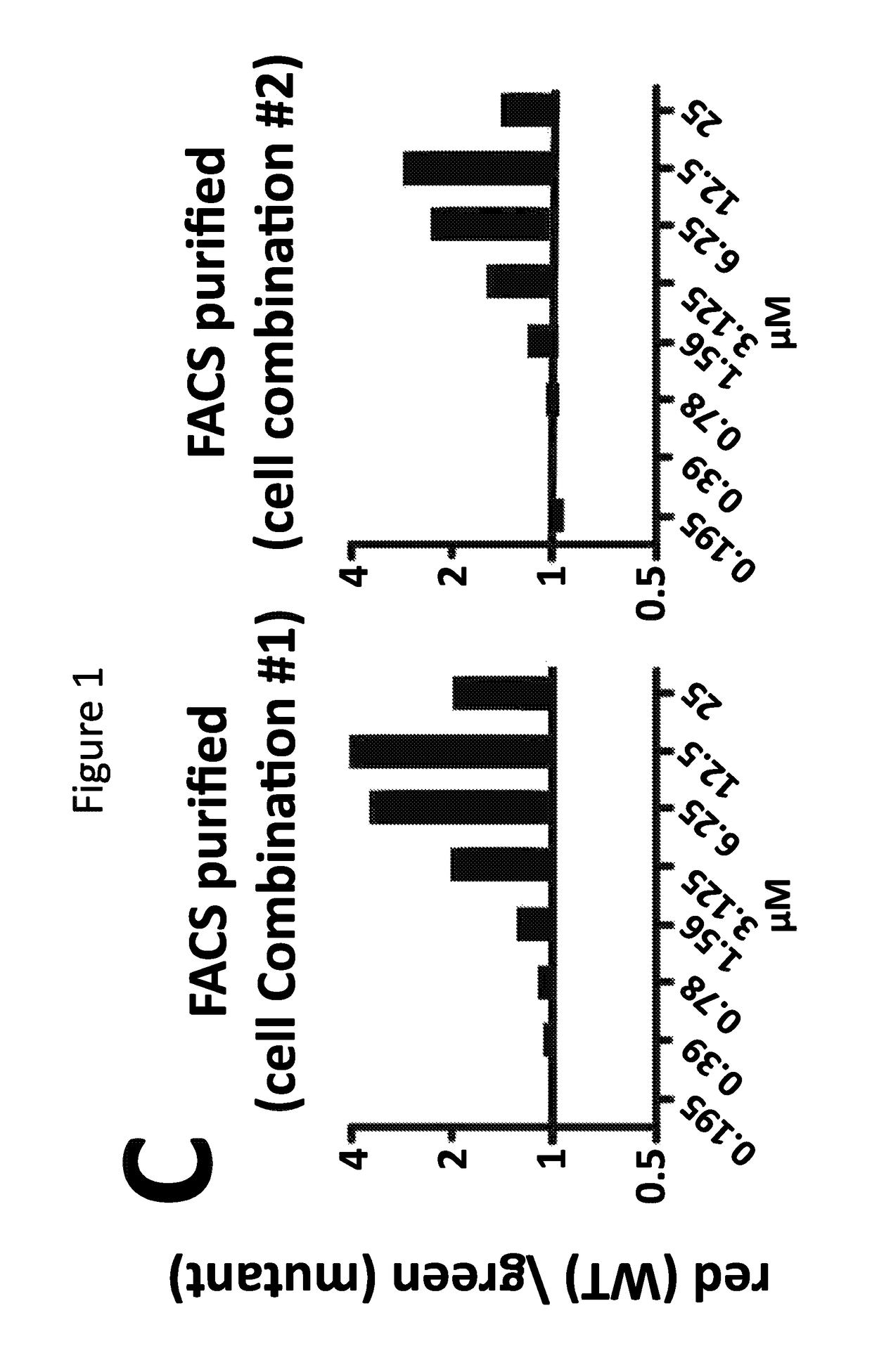
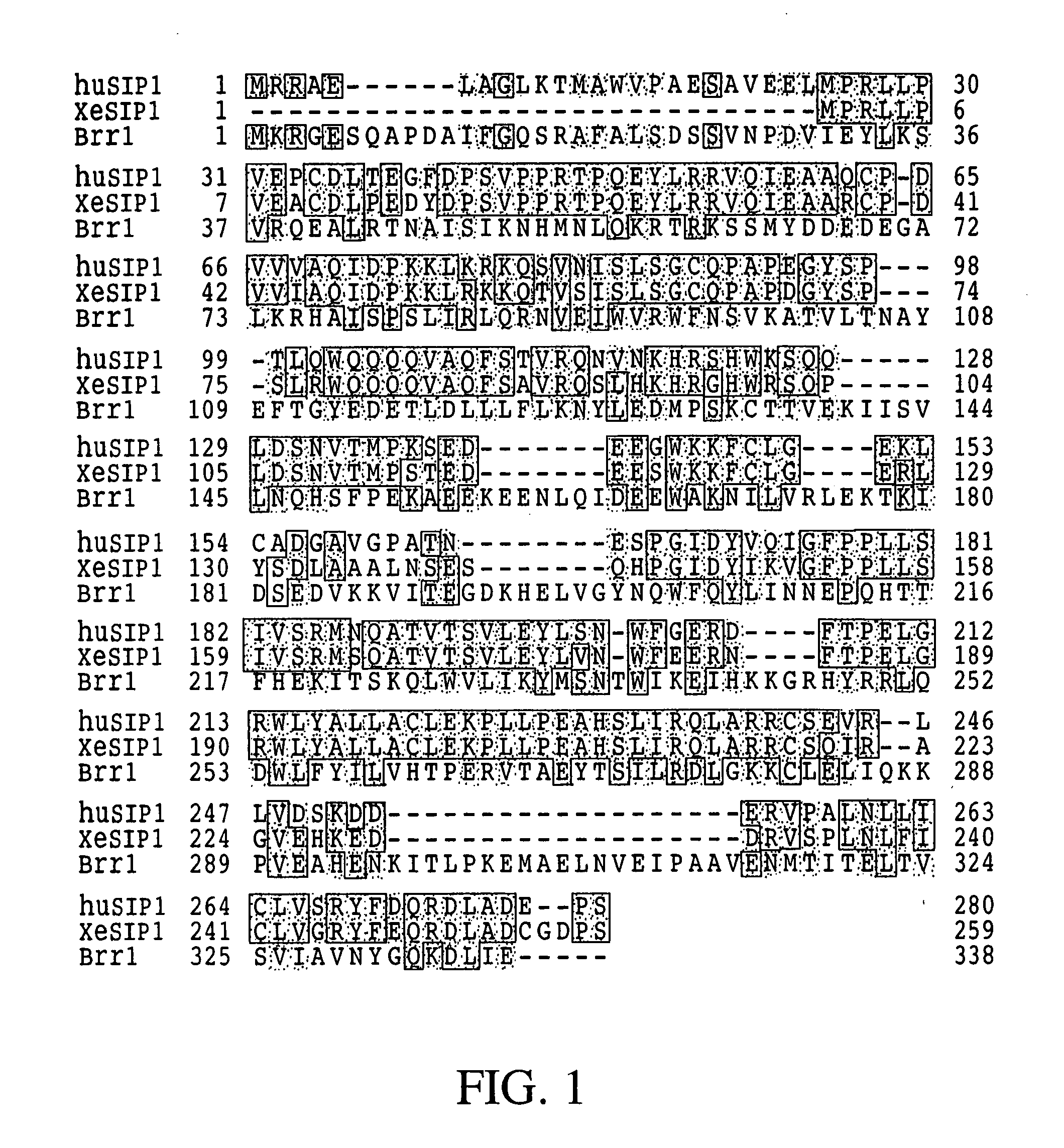
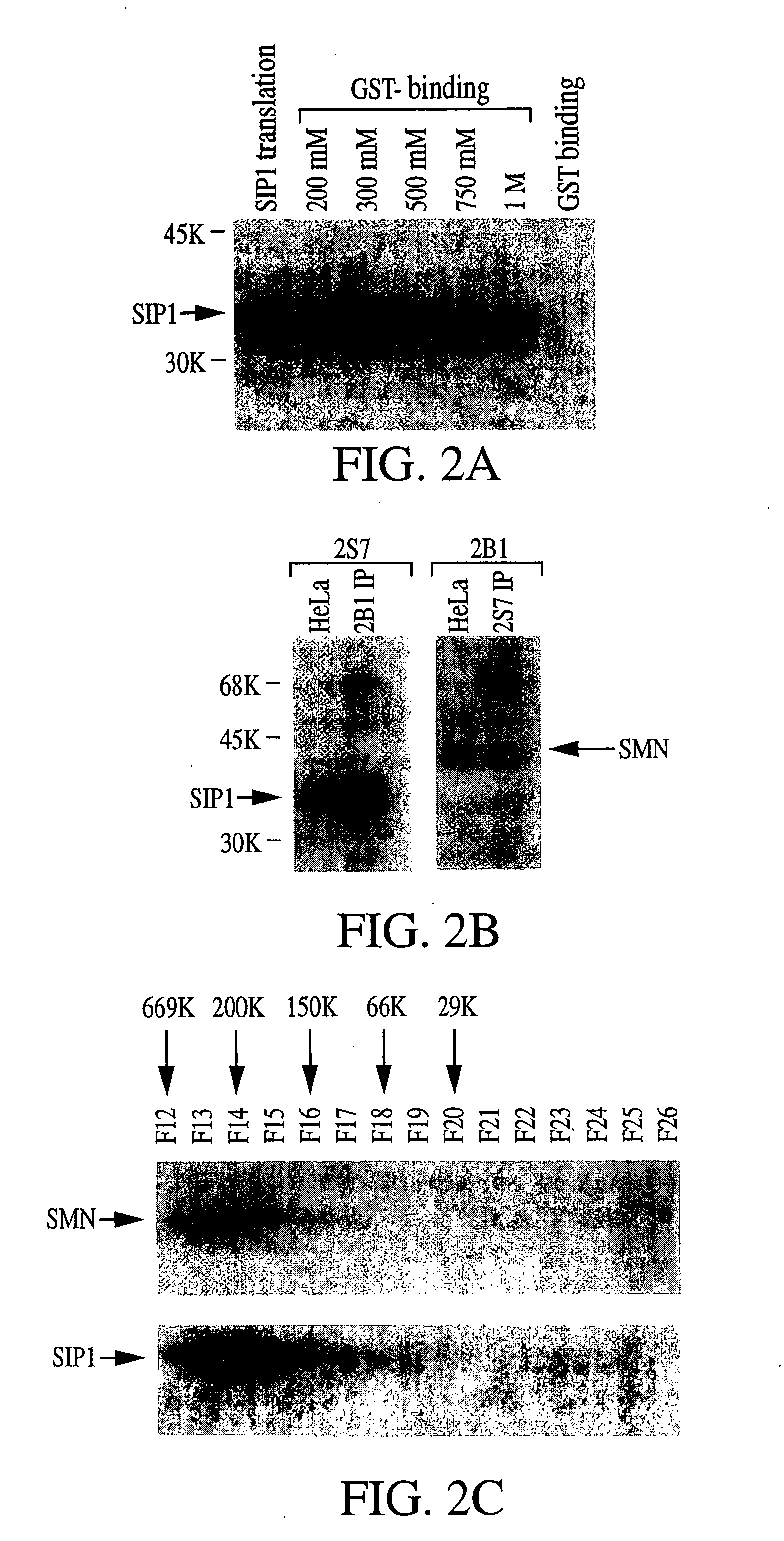
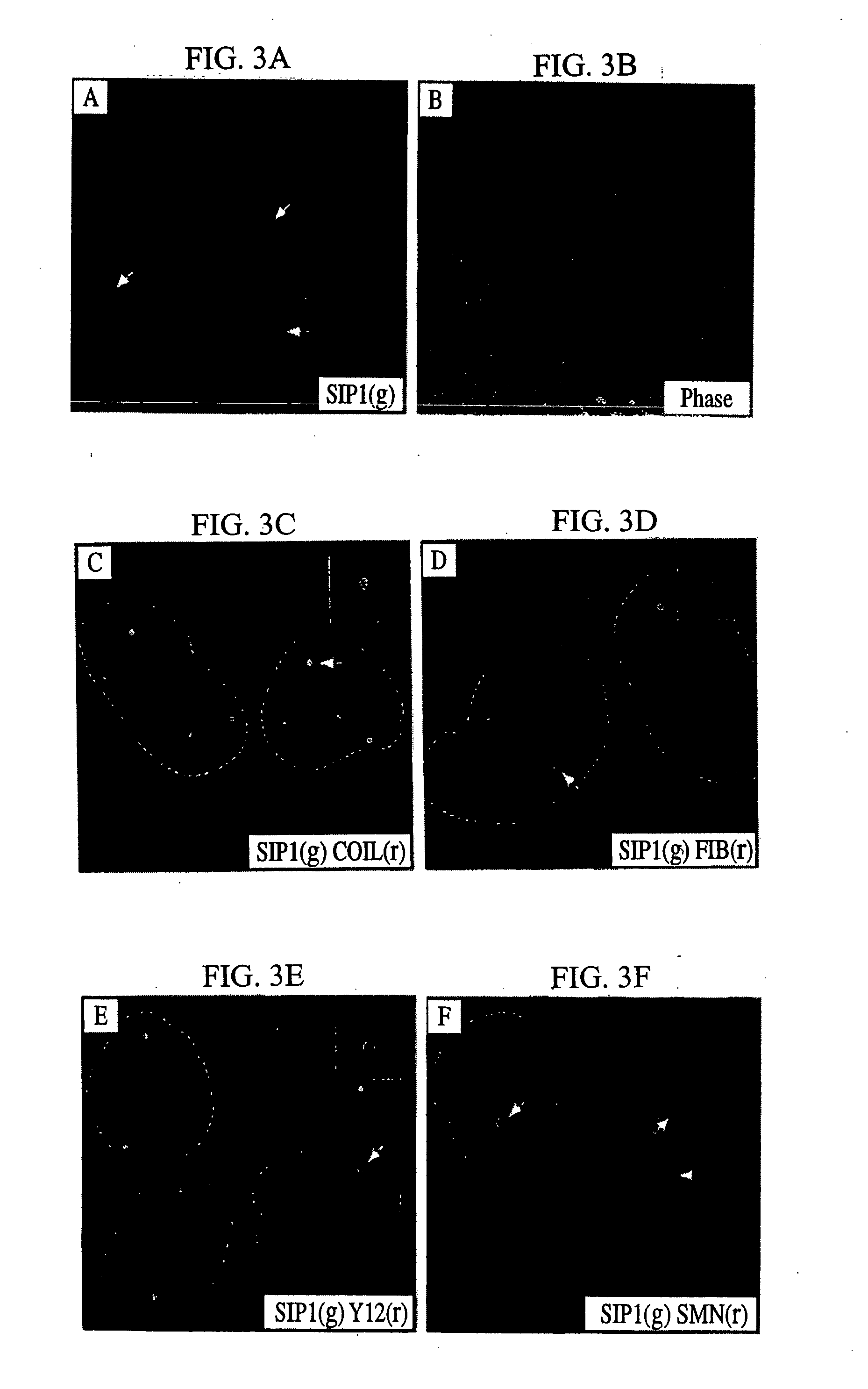
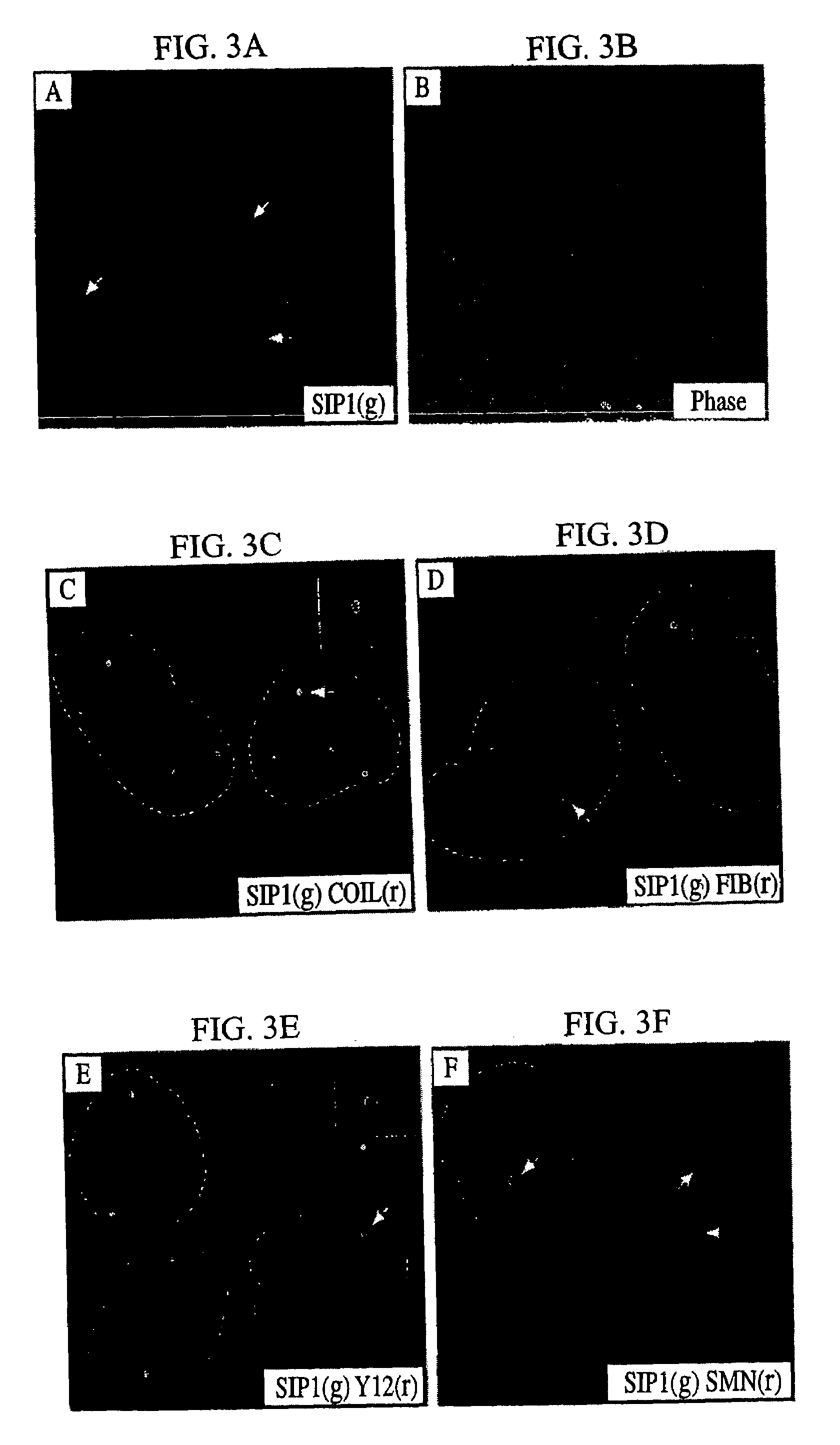
The invention relates to an isolated nucleic acid encoding a eukaryotic Survival of Motor Neuron-Interacting Protein 1 (SIP1), compositions comprising SIP1 and SIP1 and the spinal muscular atrophy (SMA) disease gene product Survival of Motor Neuron protein (SMN), and diagnostic and therapeutic assays directed to SMA. The invention also relates to another protein that specifically interacts with SMN and is a component of gems, designated Gemin3, and the nucleic acid encoding the protein. Additionally, the invention relates to a novel cell line wherein the endogenous SMN genes have been deleted and where an exogenous nucleic acid encoding SMN has been inserted into the cell such that expression of SMN in the cell is under the control of an inducible promoter. This novel cell line provides a stable genetic system for the study of SMA and for the development of SMA therapeutics.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Combination therapies for treatment of spinal muscular atrophy
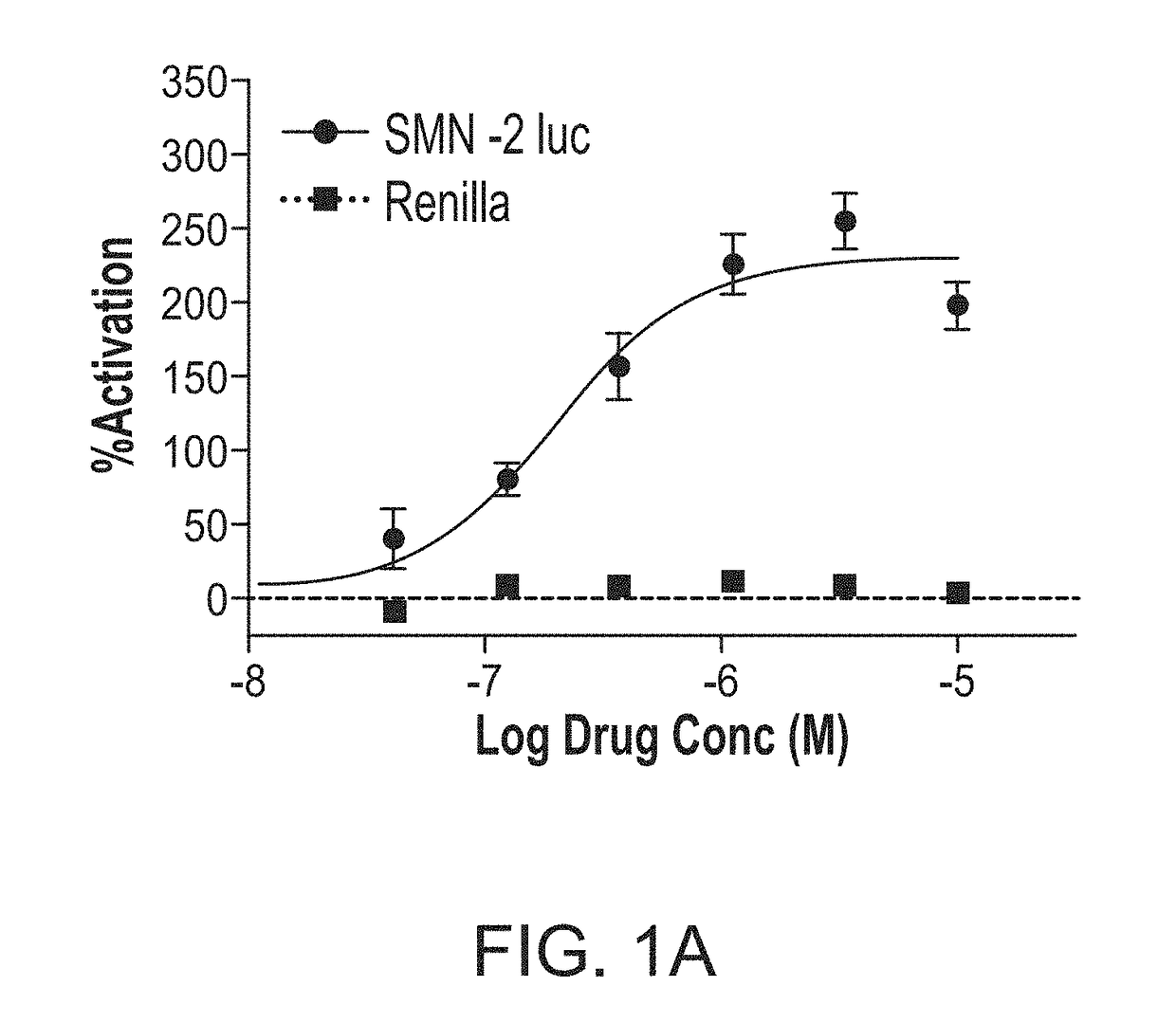
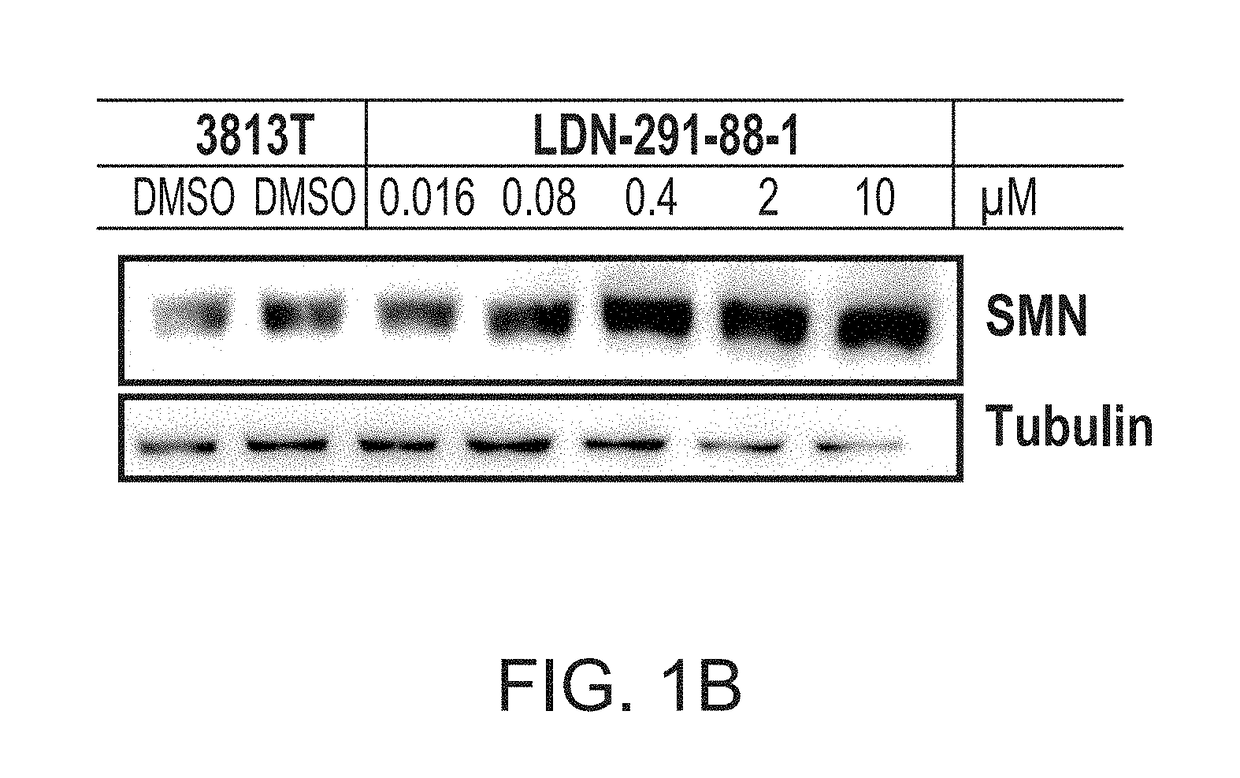
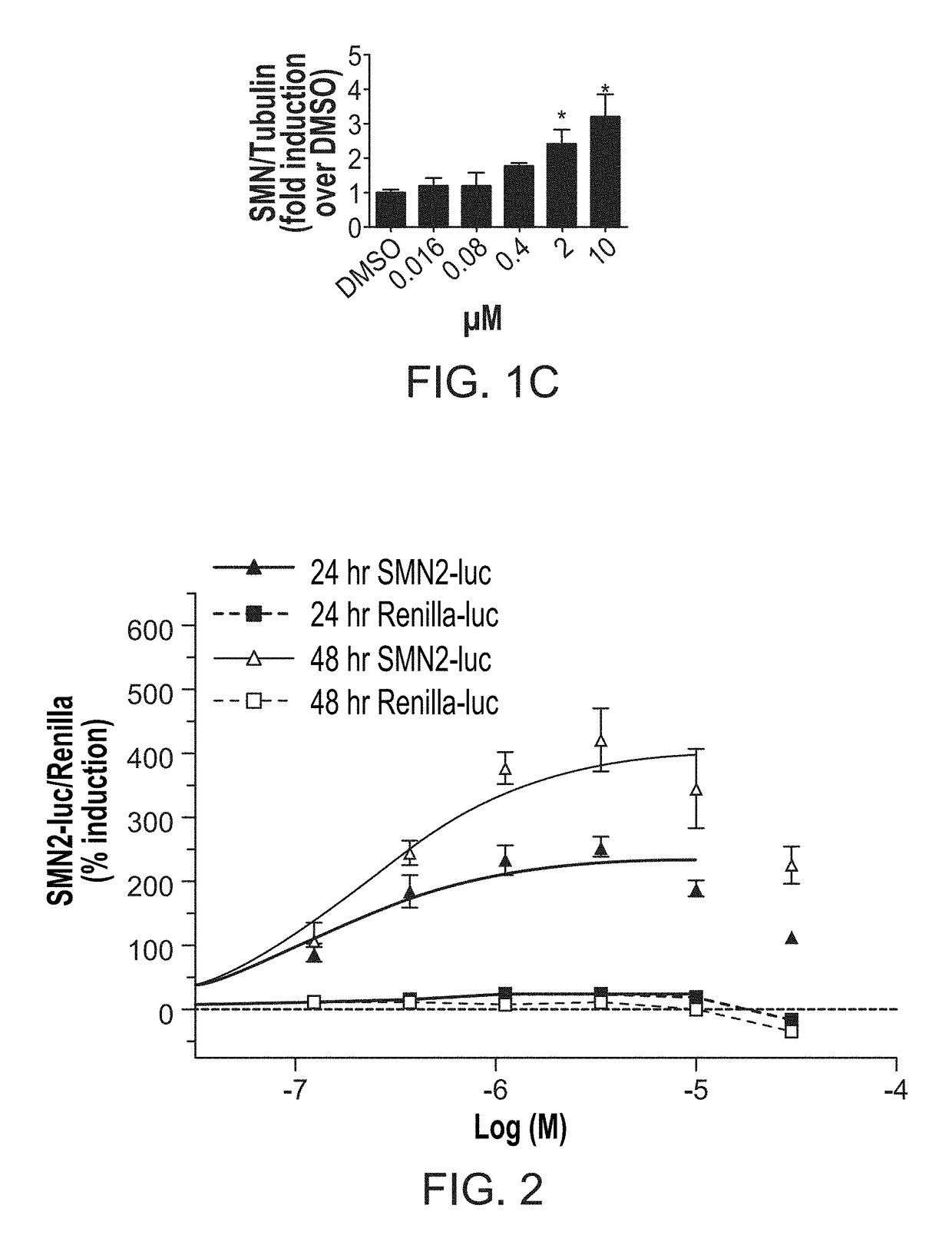
ActiveUS20170239225A1Increase full-length survivalOrganic active ingredientsMuscular disorderSurvival of motor neuronNeuron survival
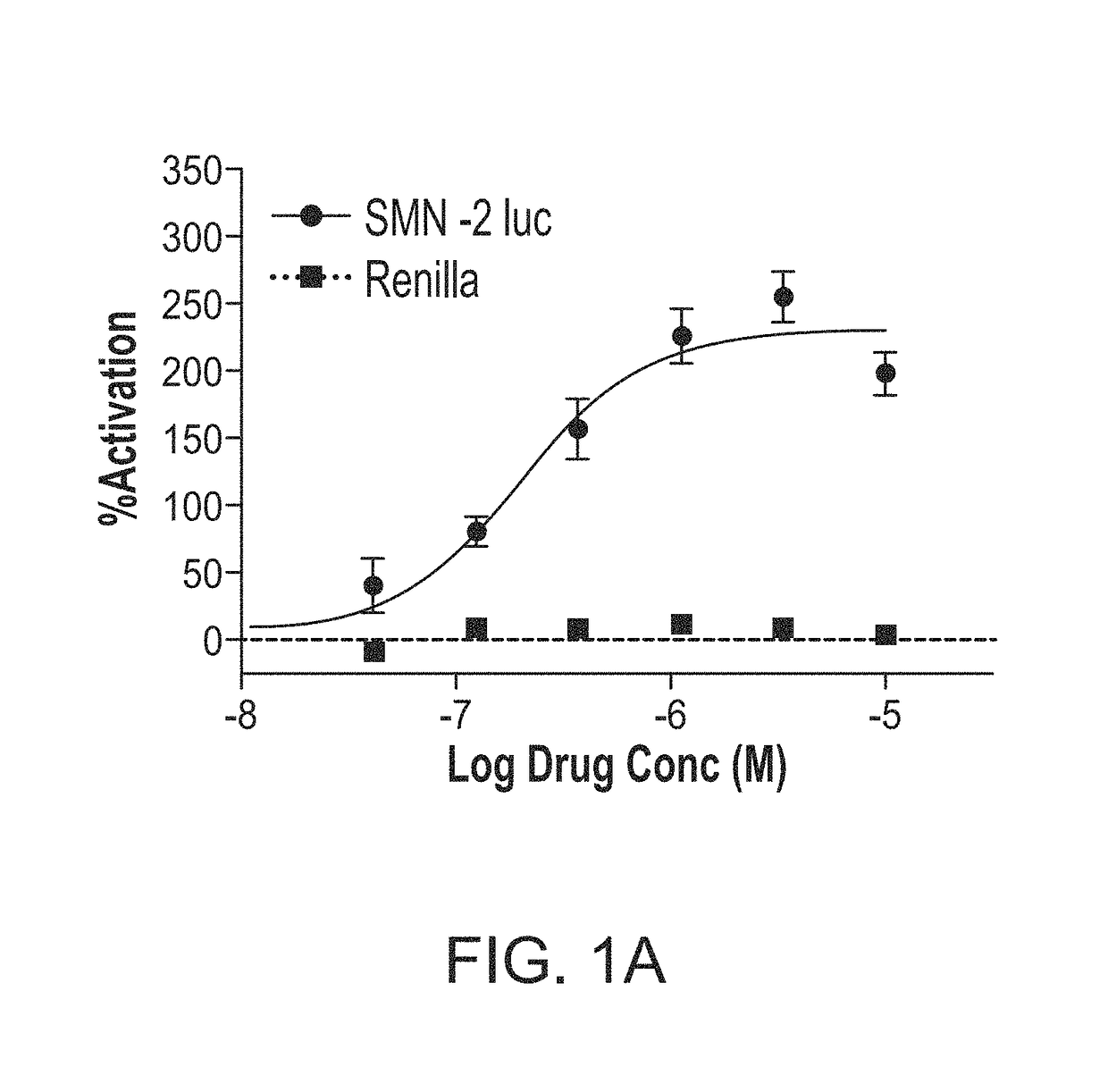
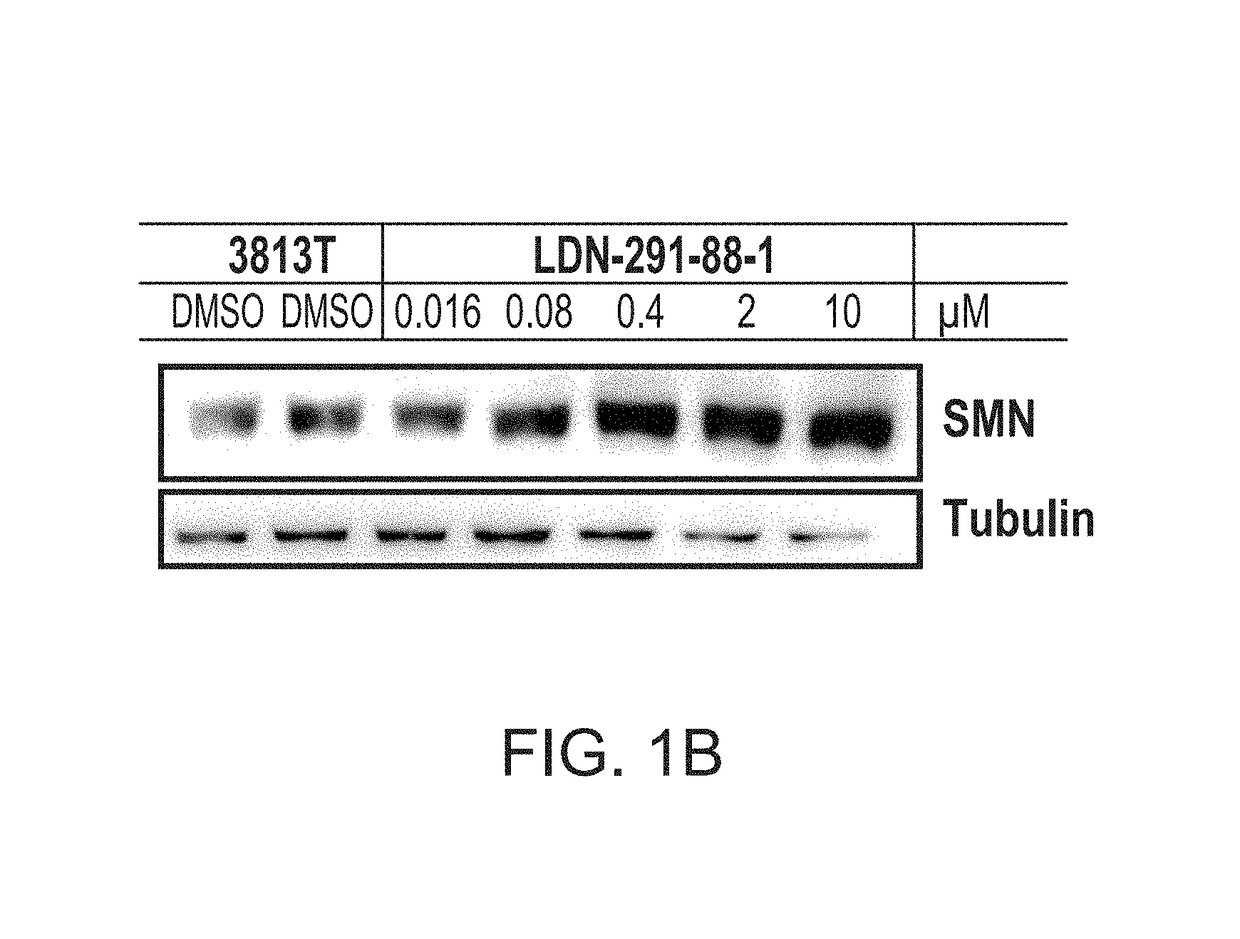
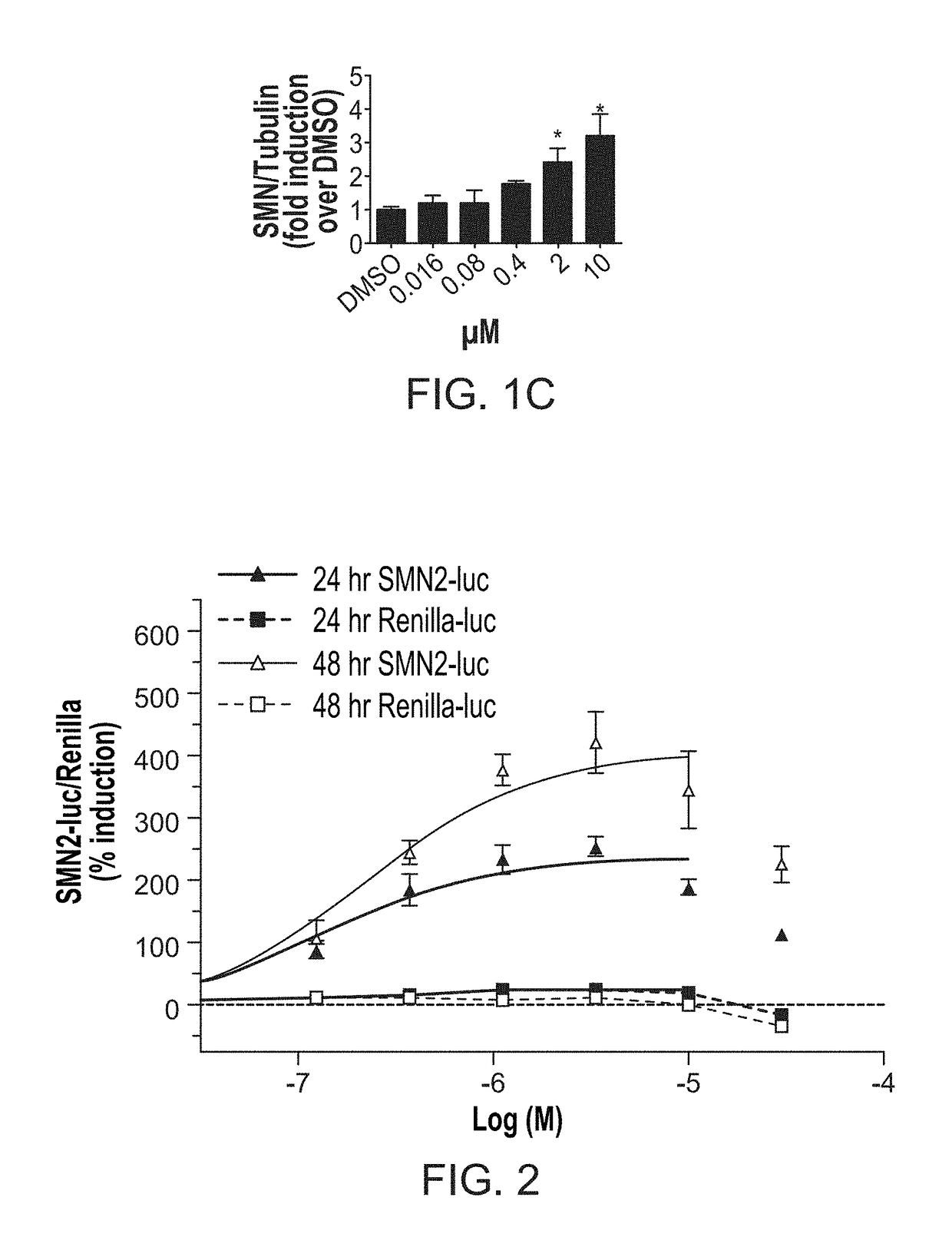
Disclosed herein are compositions and methods for treatment of spinal muscular atrophy (SMA). In certain embodiments, compounds are provided that increase full-length survival of motor neuron (SMN) protein production by an SMN2 gene.
Owner:INDIANA UNIV RES & TECH CORP +1
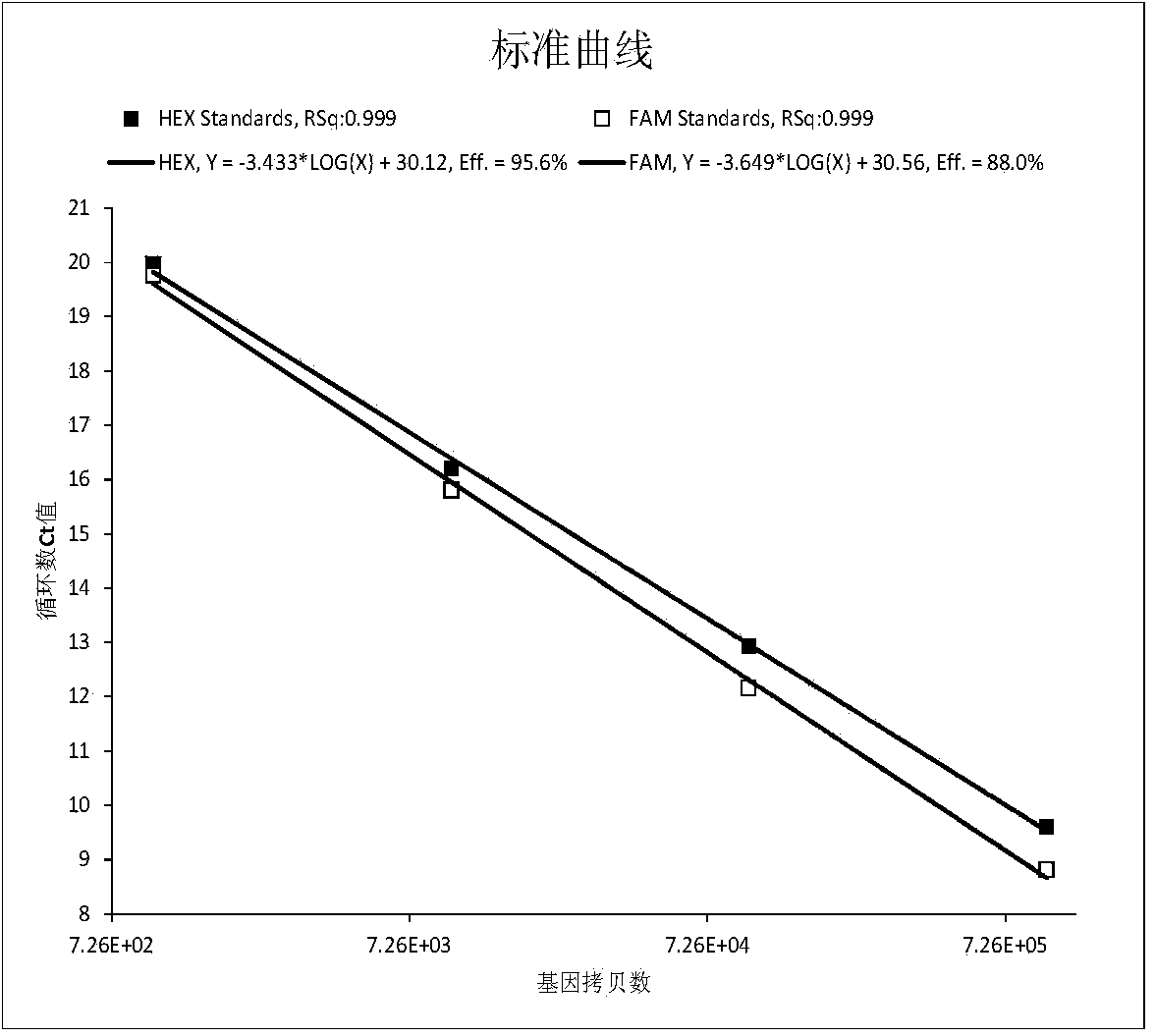
Relative quantifying kit used for detecting copy number of survival genes of human motor neurons
InactiveCN109554459AImprove accuracySmall sample sizeMicrobiological testing/measurementReference genesPrimary motor neuron
The invention relates to the field of genetic testing, and specifically relates to a kit used for detecting the copy number of survival genes of human motor neurons. According to the kit used for detecting the copy number of the survival genes of the human motor neurons, a multifluorescent polymerase chain reaction (PCR) method is adopted for detecting the copy number of the survival genes of thehuman motor neurons; and specific primers and probes for exons 7 and 8 of SMN1 gene, exons 7 and 8 of SMN2 gene, exon 6 of SMN gene and internal reference gene cystic fibrosis transmembrane regulator(CFTR) are separately designed. The kit is smartly designed, so that, relative quantifying can be separately performed on the copy number of the exons 7 and 8 of the SMN1 gene, the exons 7 and 8 of the SMN2 gene as well as the exon 6 of the SMN gene by adopting 3 independent PCR reactions. The kit is easy to operate, strong in specificity and excellent in sensitivity. Thus, the result of the copynumber of the exon 6 of the SMN gene can be improved in terms of accuracy; so that, the kit is more suitable for clinical classifying of patients with spinal muscular atrophy and screening of carriers.
Owner:DEBIQI BIOTECH XIAMEN
Isolated polypeptide deletion mutants of survival of motor neuron-interacting protein 1
InactiveUS7459309B2Improve the level ofEasy splicingNanotechBacteriaSurvival of motor neuronNeuron survival
The invention relates to an isolated nucleic acid encoding a eukaryotic Survival of Motor Neuron-Interacting Protein 1 (SIP1), compositions comprising SIP1 and SIP1 and the spinal muscular atrophy (SMA) disease gene product Survival of Motor Neuron protein (SMN), and diagnostic and therapeutic assays directed to SMA. The invention also relates to another protein that specifically interacts with SMN and is a component of gems, designated Gemin3, and the nucleic acid encoding the protein. Additionally, the invention relates to a novel cell line wherein the endogenous SMN genes have been deleted and where an exogenous nucleic acid encoding SMN has been inserted into the cell such that expression of SMN in the cell is under the control of an inducible promoter. This novel cell line provides a stable genetic system for the study of SMA and for the development of SMA therapeutics.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Combination therapies for treatment of spinal muscular atrophy
ActiveUS9895358B2Increase full-length survivalMuscular disorderNeuromuscular disorderSurvival of motor neuronPsychiatry
Disclosed herein are compositions and methods for treatment of spinal muscular atrophy (SMA). In certain embodiments, compounds are provided that increase full-length survival of motor neuron (SMN) protein production by an SMN2 gene.
Owner:INDIANA UNIV RES & TECH CORP +1
ADP-ribosyl transferase fusion variant proteins
InactiveUS7795218B2Inhibition of uncontrolledPrevention of uncontrolledSenses disorderNervous disorderNervous systemNeuron survival
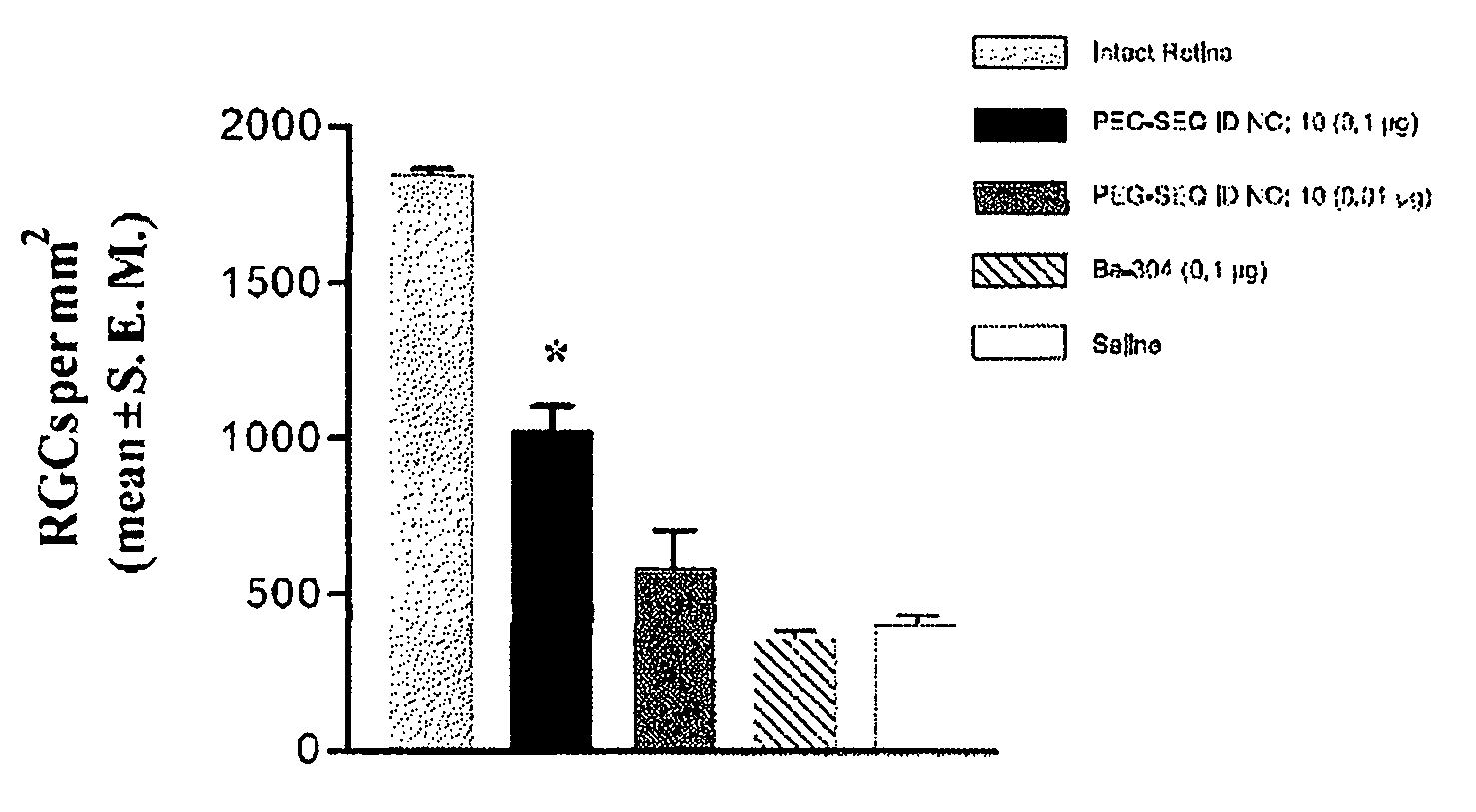
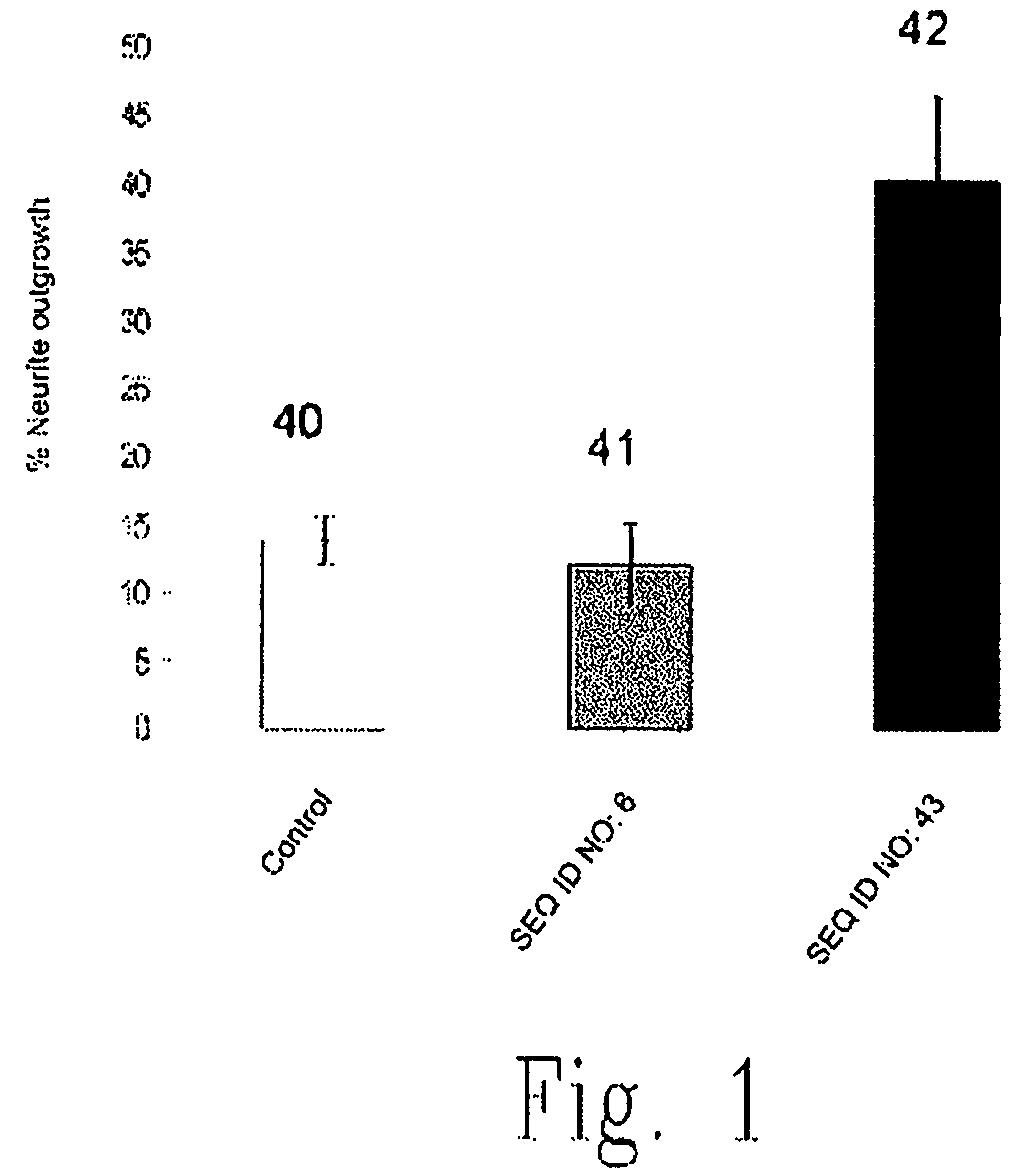
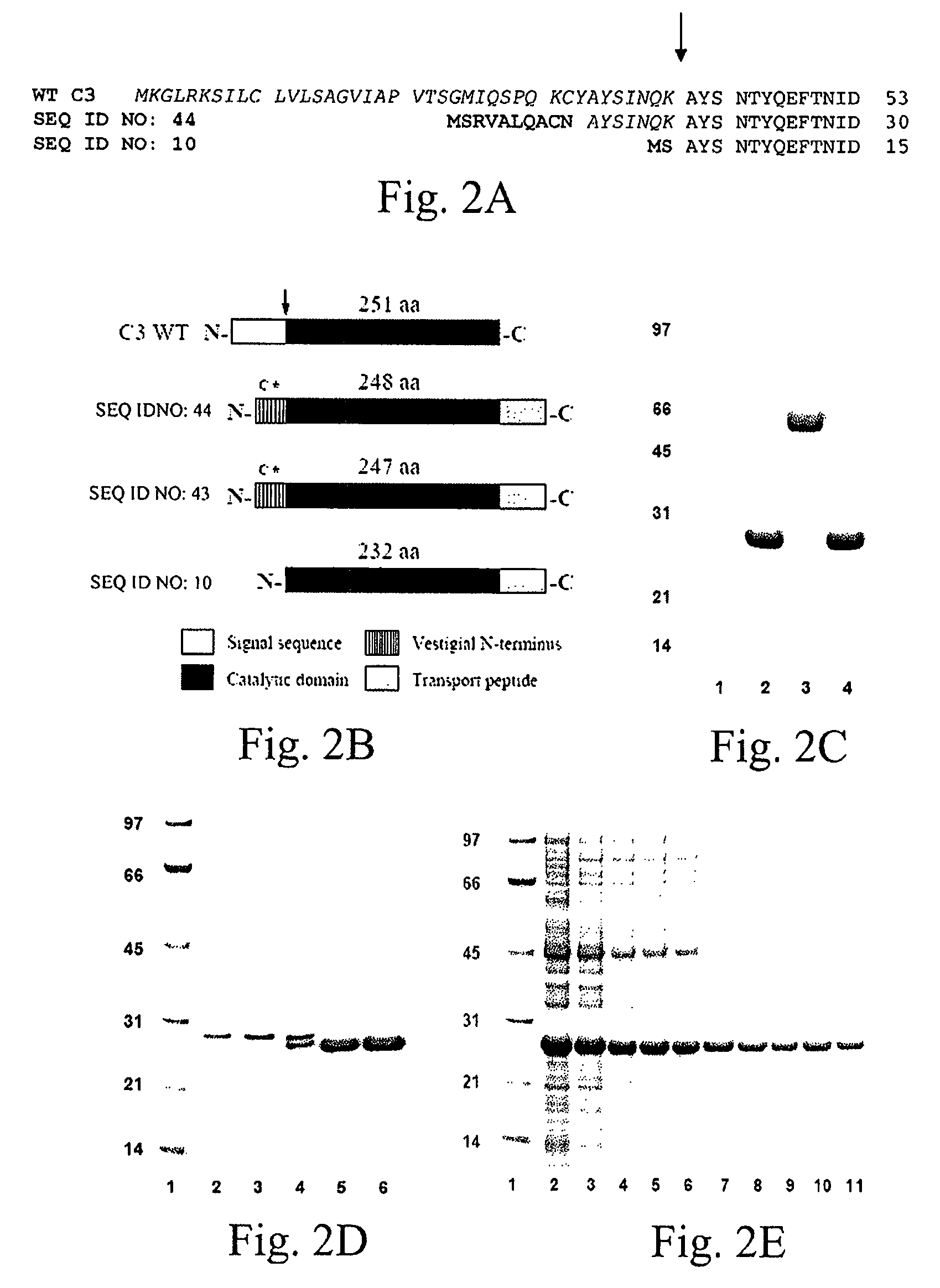
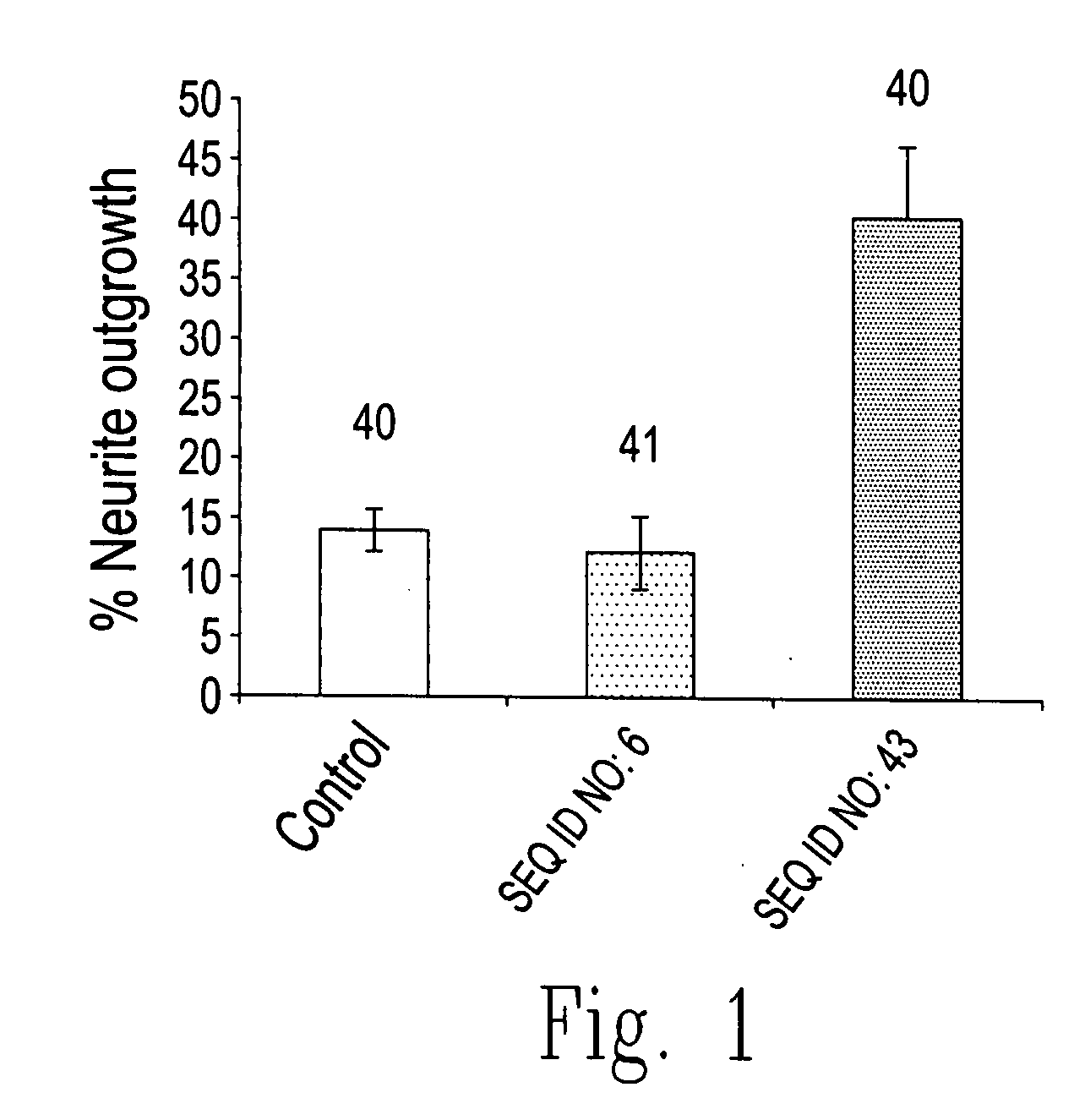
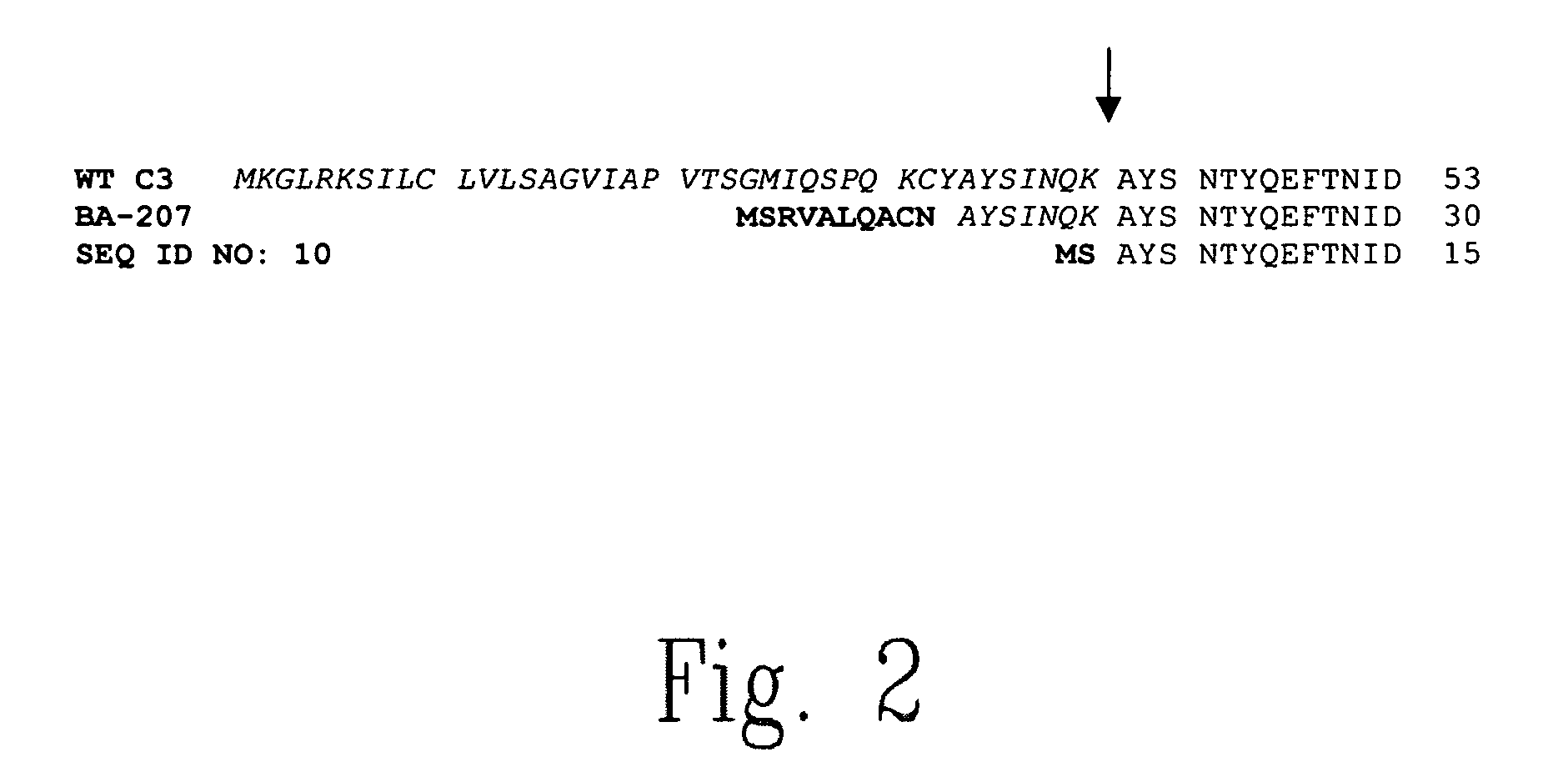
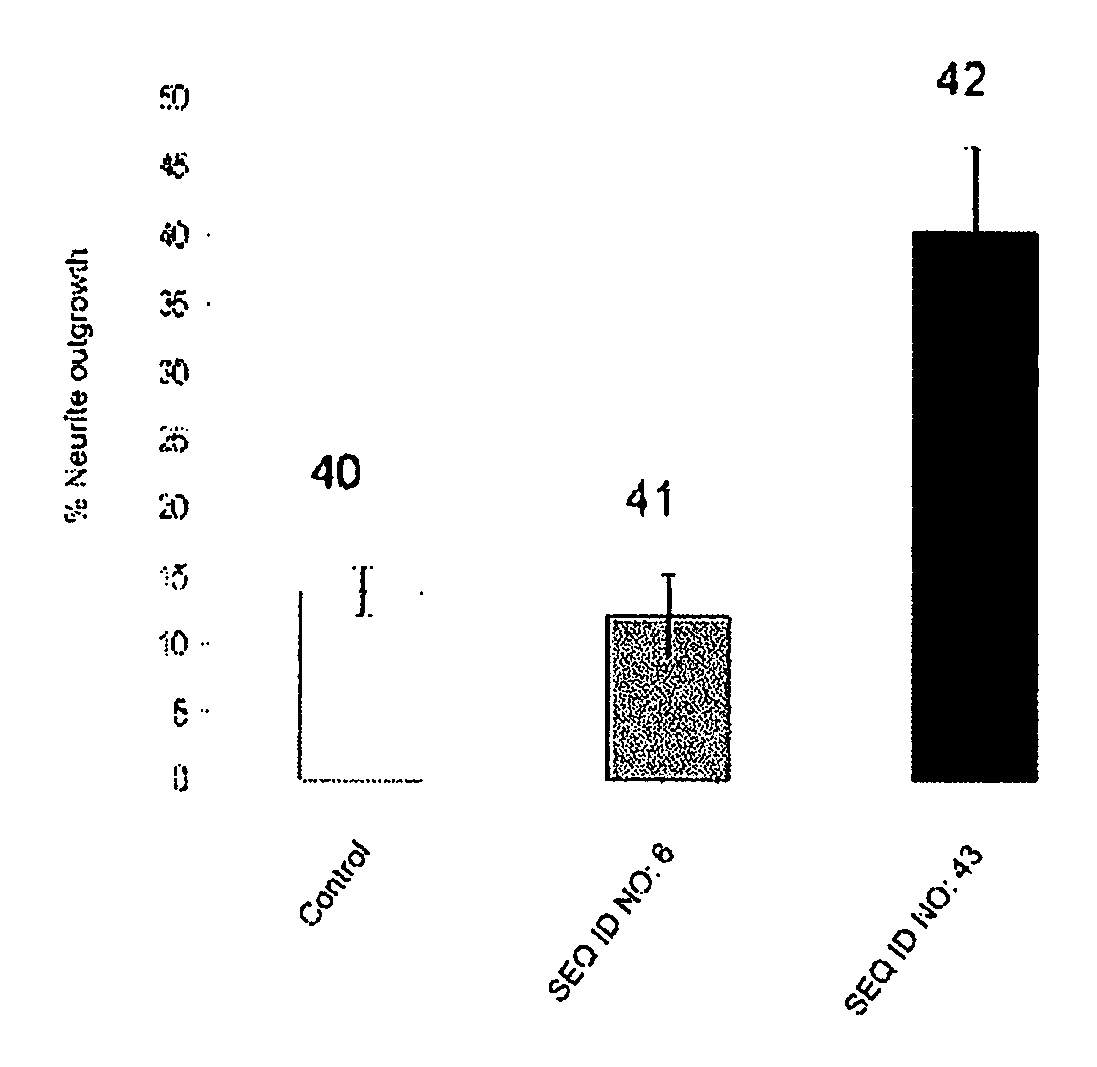
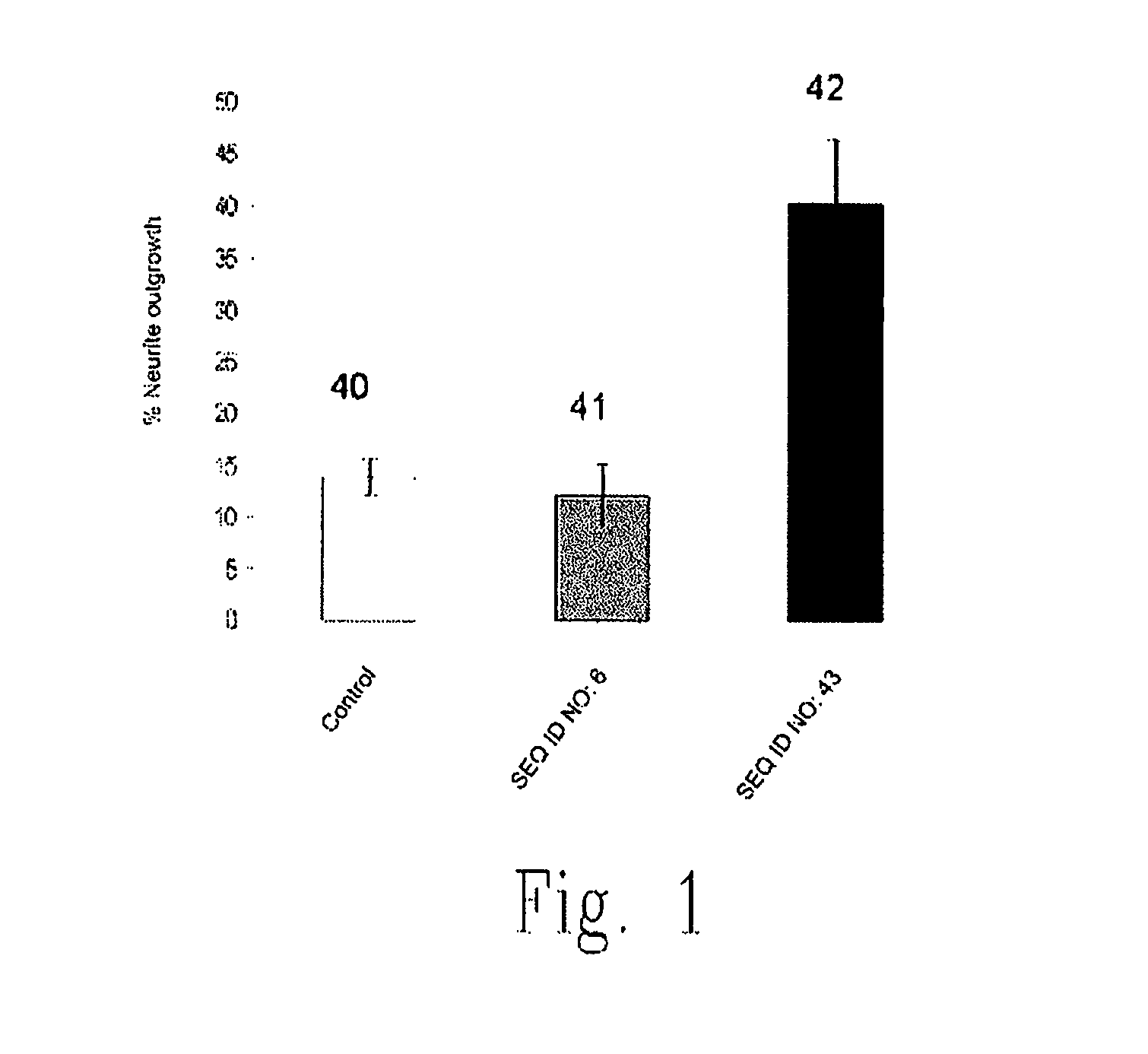
The present invention relates to novel chimeric C3-like Rho antagonists and their use for promoting repair and neuron survival in injured mammalian central and peripheral nervous system and for treating or preventing cancer.
Owner:BIOAXONE BIOSCI
Treatment of parkinsons disease with glycolipids
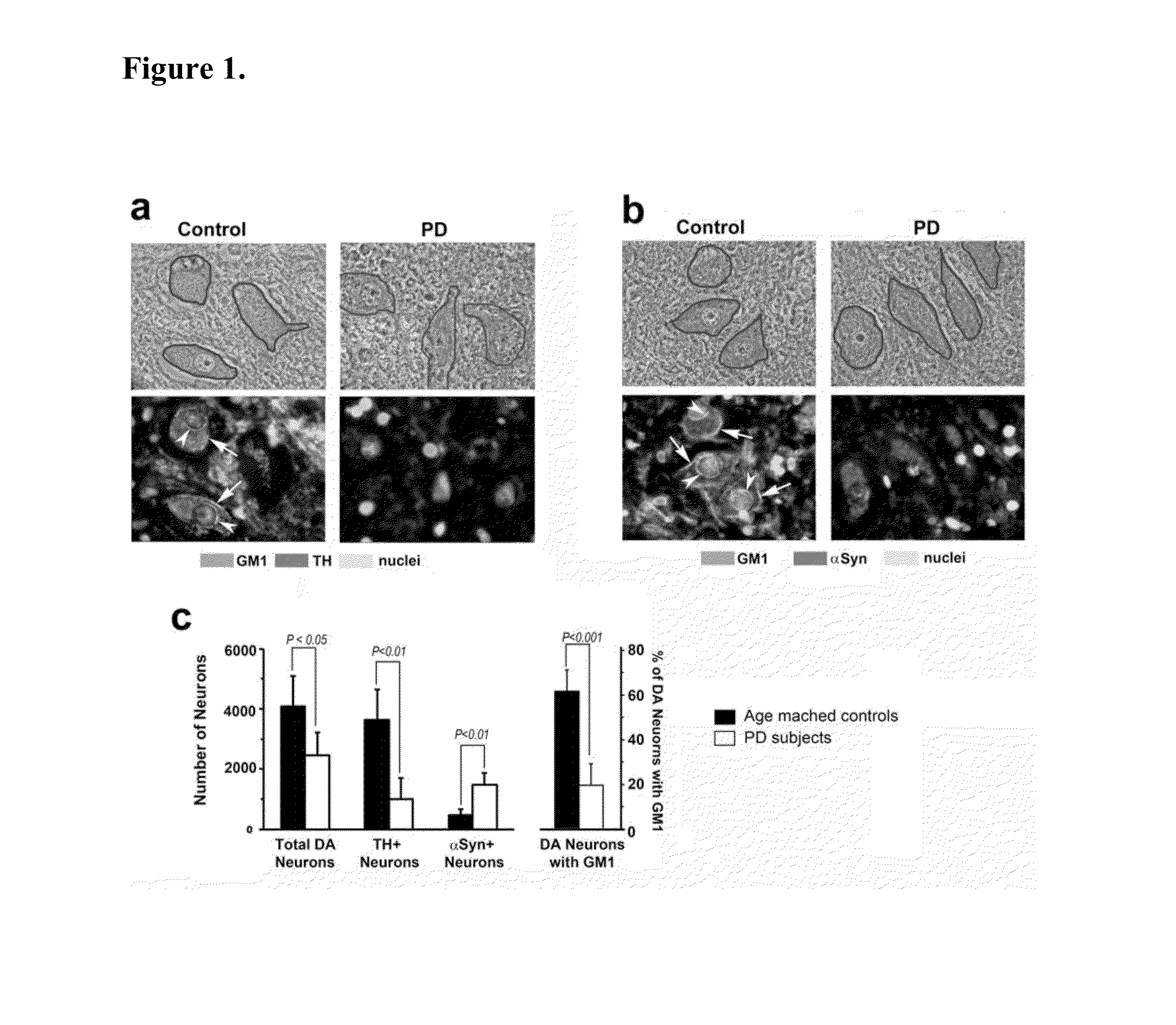
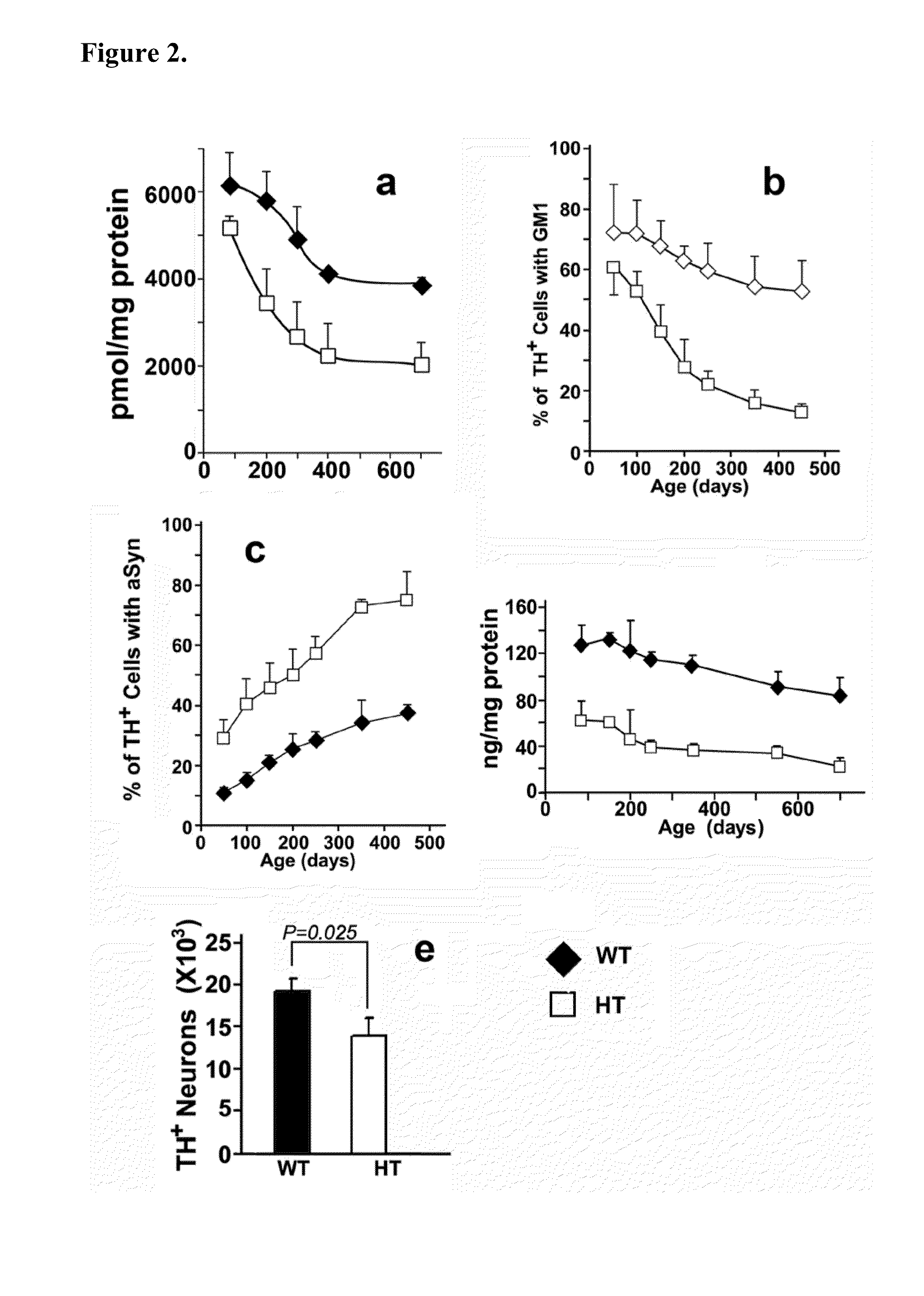
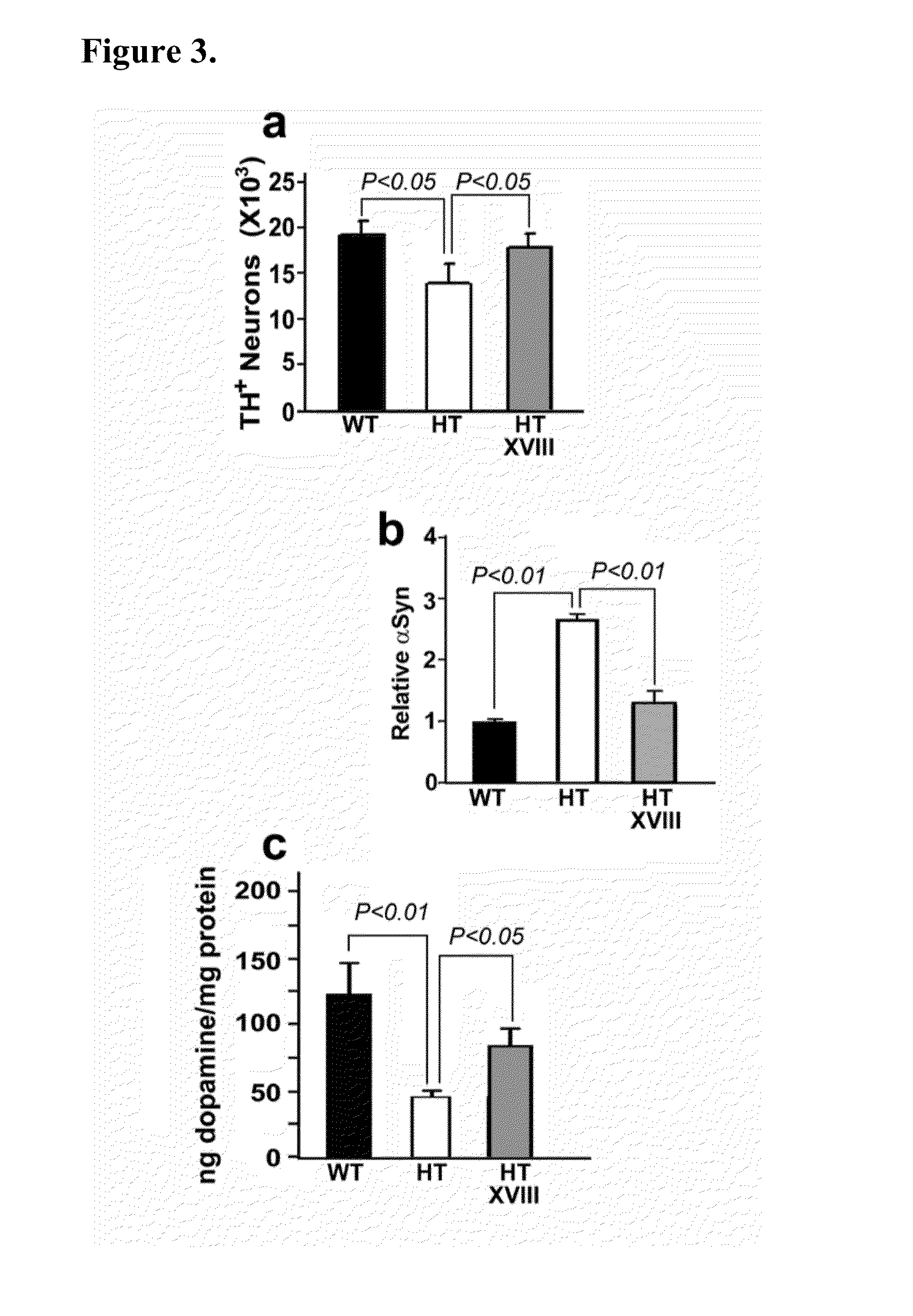
InactiveUS20140187507A1Reduced activityIncrease RET activityBiocideSugar derivativesNeuron cell deathMedicine
This invention provides compounds, compositions, and methods for treating Parkinson's disease. In particular, there is provided GM1 ganglioside analogs that are capable of penetrating the blood brain barrier and entering the cytoplasm of neurons. Endogenous GM1 interacts with the with GFRα1 / RET, the GDNF receptor complex involved in GDNF signaling which is essential for neuron survival. In neurons that are deficient in endogenous GM1, the GM1 analog compounds of the invention are capable of restoring GDNF signaling thereby preventing neuron cell death.
Owner:RUTGERS THE STATE UNIV
Detection kit for human survival motor neuron genes SMN1 and SMN2, and detection method
PendingCN106520942AImprove featuresHigh sensitivityMicrobiological testing/measurementReference genesFluorescence
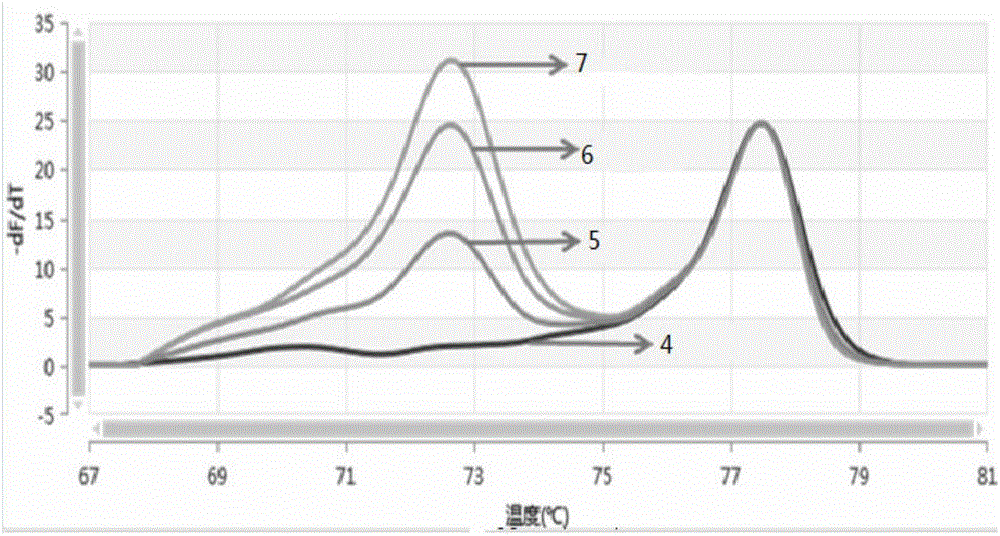
The invention discloses a detection kit for human survival motor neuron genes SMN1 and SMN2, and a method. The kit comprises an SMN1 main reaction liquid, an SMN2 main reaction liquid, an enzyme mixed liquid, 0-copy quality control, SMN1 single-copy quality control, SMN1 two-copy quality control, SMN2 single-copy quality control and SMN2 two-copy quality control, wherein the SMN1 main reaction liquid comprises a detection primer of the gene SMN1 and an inner reference primer of the SMN1; and the SMN2 main reaction liquid comprises a detection primer of the gene SMN2 and an inner reference primer of the SMN2. According to the kit, a PCR melting curve method is adopted, the change of a fluorescence signal value during a double-strand DNA melting process is detected in real time, target genes and inner reference genes generate different dissolution peaks according to the difference of different amplification product quantities, and then the copy numbers of the genes SMN1 and SMN2 are judged through software processing. The kit and the method which are disclosed by the invention are high in detection result accuracy, high in sensitivity, less in detection time consumption and low in cost.
Owner:苏州天隆生物科技有限公司
Screening methods for spinal muscular atrophy
ActiveUS20140193906A1Increase full-length survivalHigh expressionSugar derivativesMuscular disorderSurvival of motor neuronAnesthesia
Disclosed herein are compositions and methods for treatment of spinal muscular atrophy (SMA). In certain embodiments, compounds are provided that increase full-length survival of motor neuron (SMN) protein production by an SMN2 gene.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +2
ADP-ribosyl transferase fusion variant proteins
InactiveUS20070270340A1Uncontrolled proliferationInhibiting and reducingAntibacterial agentsBiocideCancer preventionNervous system
The present invention relates to novel chimeric C3-like Rho antagonists and their use for promoting repair and neuron survival in injured mammalian central and peripheral nervous system and for treating or preventing cancer.
Owner:BIOAXONE BIOSCI
ADP-ribosyl transferase fusion variant proteins
InactiveUS20080269120A1Uncontrolled proliferationInhibiting and reducingSenses disorderNervous disorderCancer preventionNervous system
The present invention relates to novel chimeric C3-like Rho antagonists and their use for promoting repair and neuron survival in injured mammalian central and peripheral nervous system and for treating or preventing cancer.
Owner:BIOAXONE BIOSCI
Compounds, compositions and methods for treating or preventing neurodegenerative disorders
InactiveUS20150164901A1Inhibitory activityLevel avoidBiocideOrganic active ingredientsMedicineAmyotrophic lateral sclerosis
The disclosure provides compounds, compositions, and methods for promoting motor neuron survival, and for treating or preventing neurodegenerative disorders, such as spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS).
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
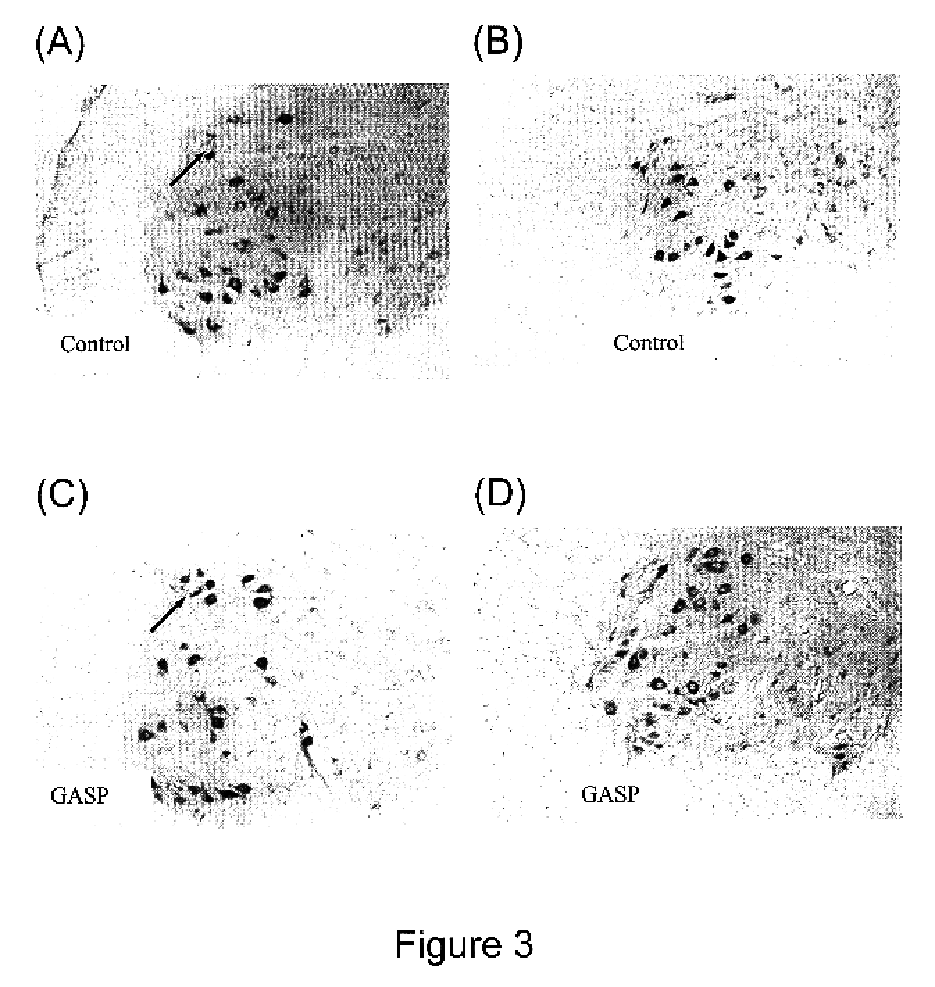
Effects of sporoderm-broken germination activated ganoderma spores on treatment of spinal cord injury
The present invention provides a method for treating patients with spinal cord injury by administering germination activated Ganoderma lucidium spores (GASP) to the patients. The GASP are sporoderm-broken. The GASP promote axon regeneration and improve motor neuron survival in the injured spinal cord.
Owner:ENHAN TECH HLDG INT
Co-activation of mTOR and STAT3 pathways to promote neuronal survival and regeneration
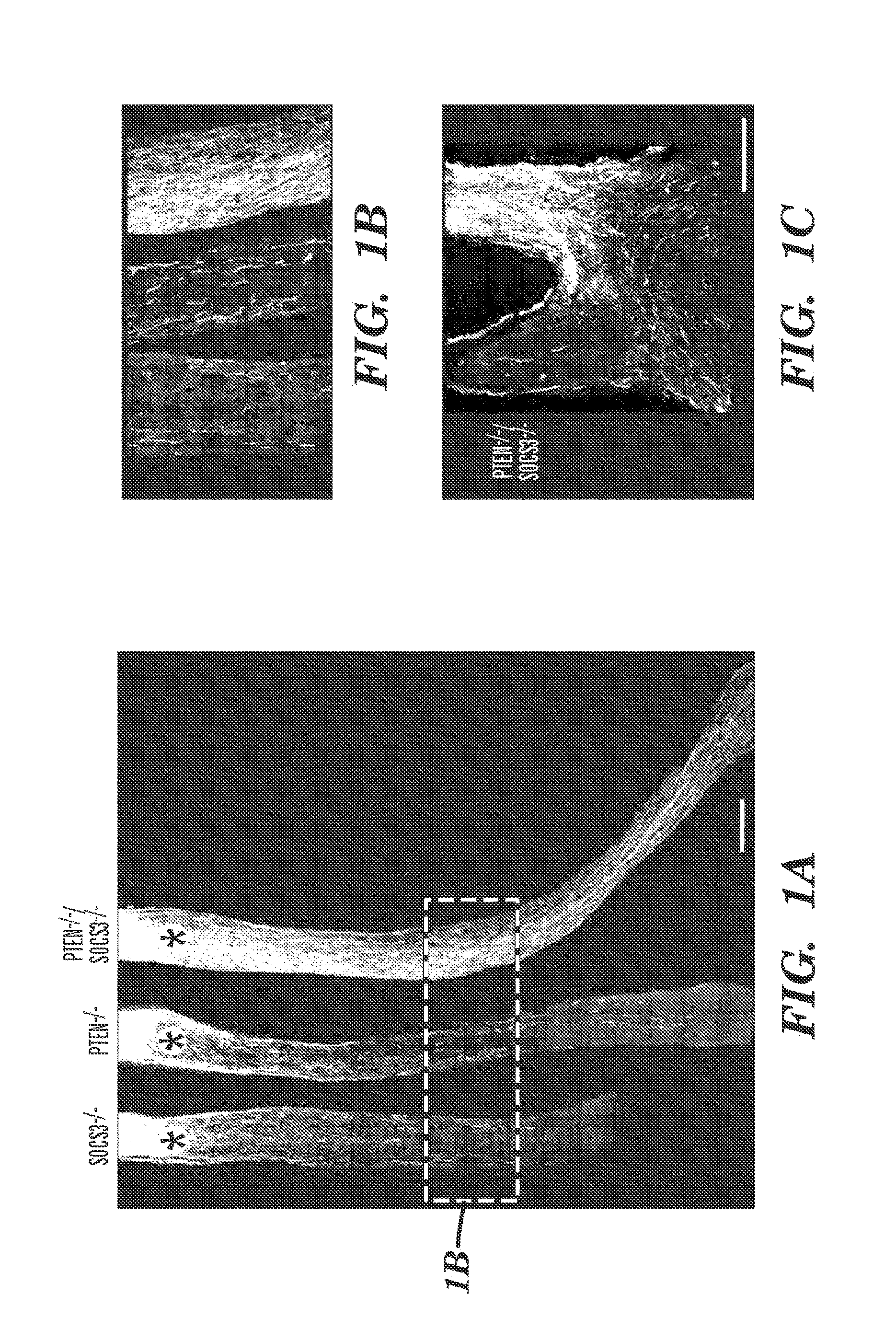
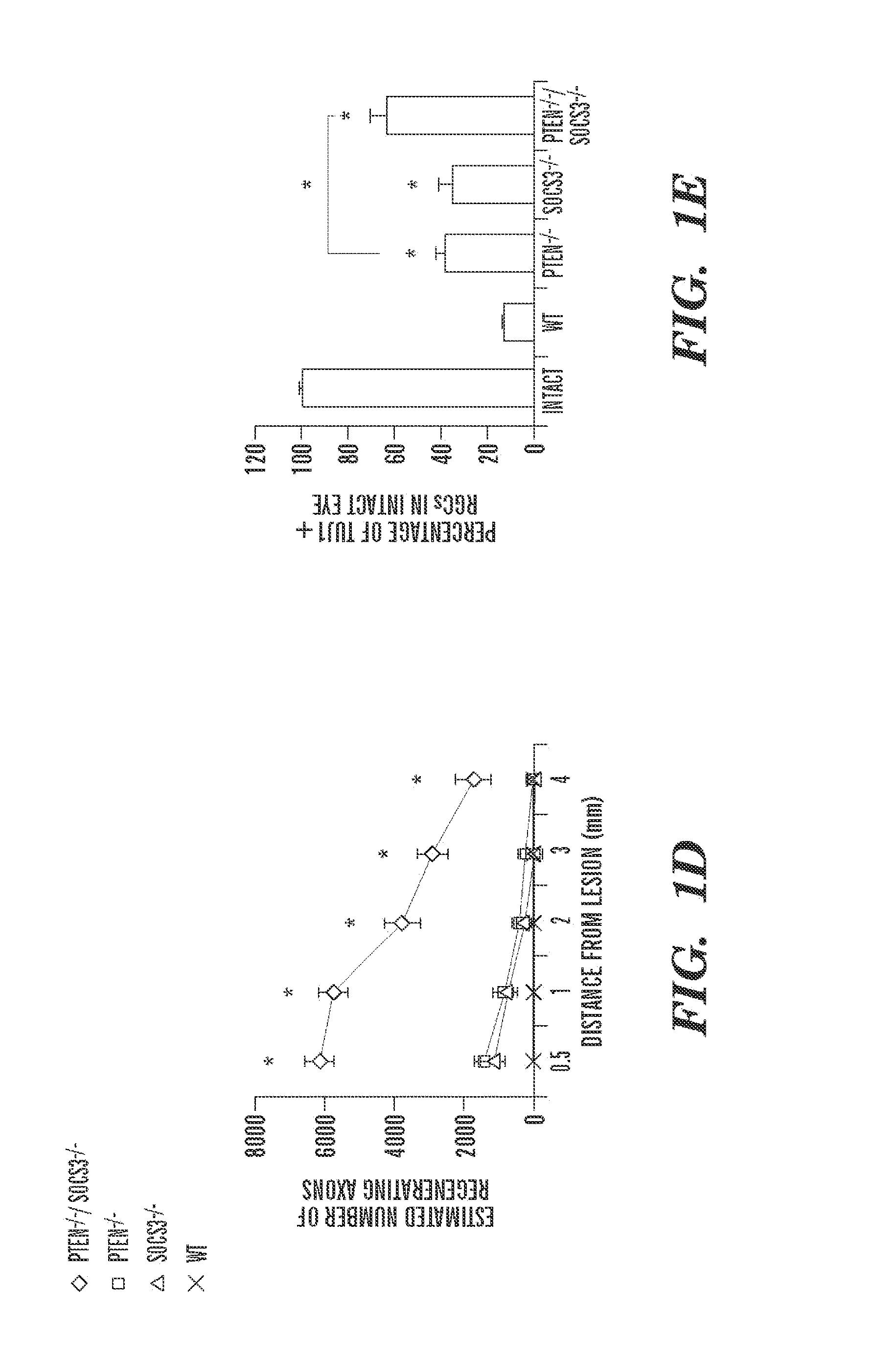
Disclosed herein is a method of promoting sustained survival, sustained regeneration, in a lesioned mature neuron, sustained compensatory outgrowth in a neuron, or combinations thereof. The method comprises contacting the lesioned mature neuron with an effective amount of an inhibitor of PTEN and an effective amount of an inhibitor of SOCS3 to thereby promote survival and / or regeneration and / or compensatory outgrowth of the neuron. Therapeutic methods of treatment of a subject with a neuronal lesion by administration of a therapeutically effective amount of an inhibitor of PTEN and a therapeutically effective amount of an inhibitor of SOCS3, are also disclosed, as are pharmaceutical compositions and devices for use in the methods.
Owner:CHILDRENS MEDICAL CENT CORP
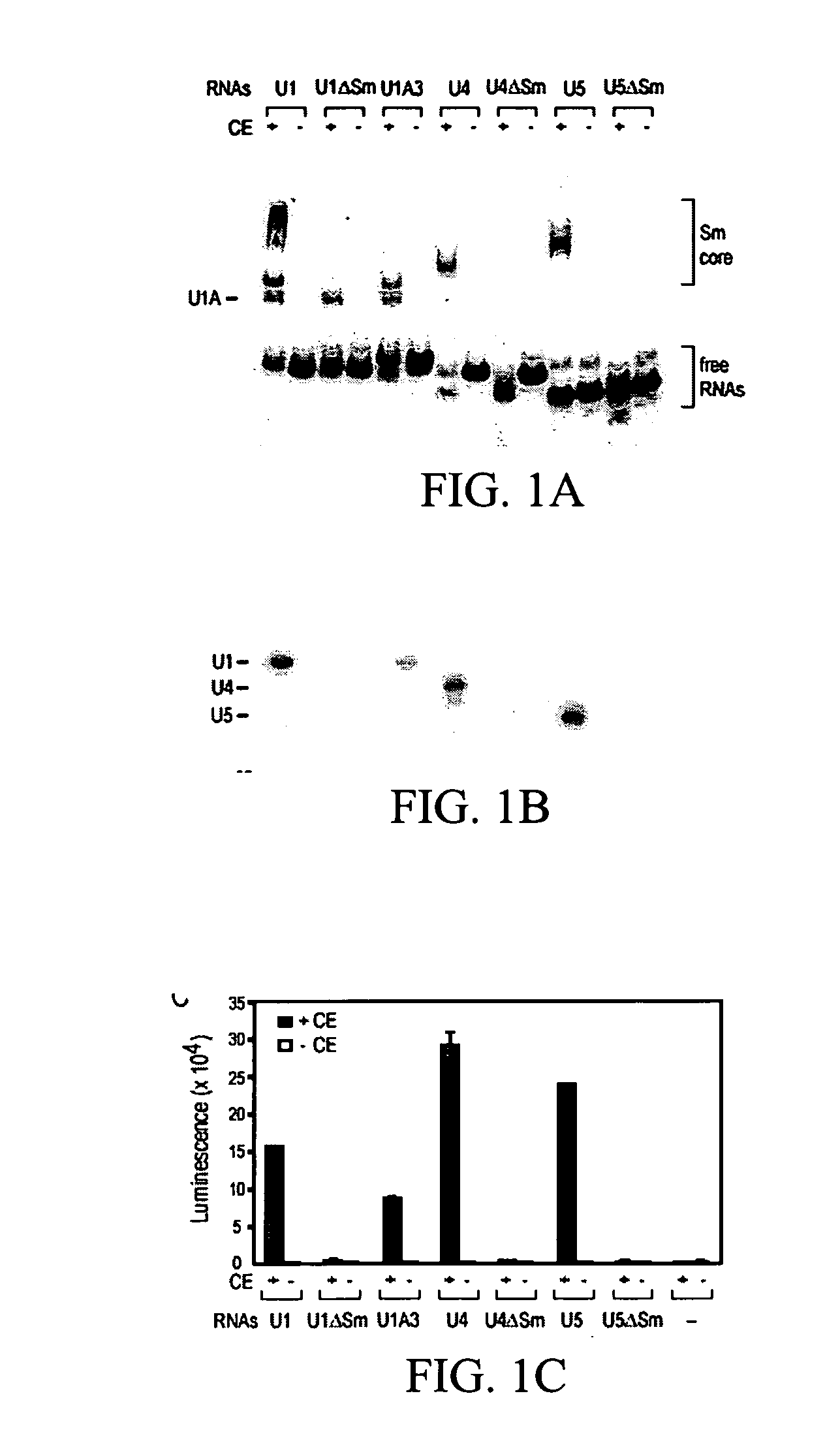
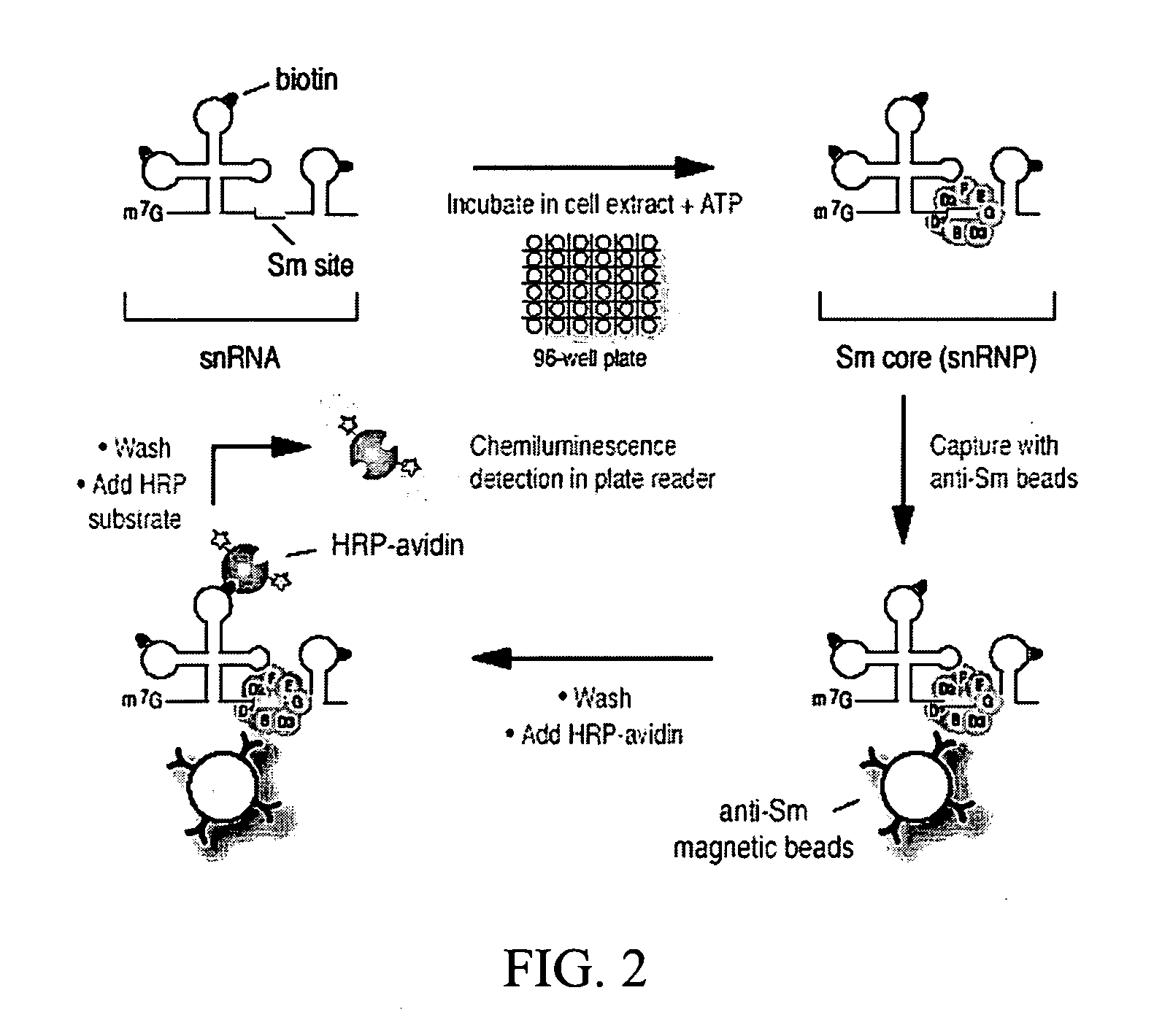
Assays, methods and kits for detecting small nuclear ribonucleoprotein particle assembly and survival of motor neurons activity
ActiveUS20060223092A1Improve the level ofDecrease in levelCompound screeningApoptosis detectionAssaySmall Nuclear Ribonucleoprotein Particle
The present invention includes assays and kits for detecting the assembly of an RNA binding protein-RNA complex and for detecting the activity of an RNA binding protein.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for promoting motor neuron survival
InactiveUS20160287602A1Organic active ingredientsMicrobiological testing/measurementSurvival of motor neuronNeuro-degenerative disease
The present invention relates to methods for promoting motor neuron survival, treating or preventing neurodegenerative disorders, identifying agents that promote survival of motor neurons, identifying agents that are useful for treating neurodegenerative disorders, diagnosing neurodegenerative disorders, predicting the progression of a neurodegenerative disorder in a subject, and monitoring the effectiveness of a therapy in reducing the progression of a neurodegenerative disorder in a subject.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Compositions and methods for improving induced neuron generation
InactiveUS20160115447A1Improve survivalAvoid signalingNervous system cellsArtificial cell constructsCell culture mediaCell type
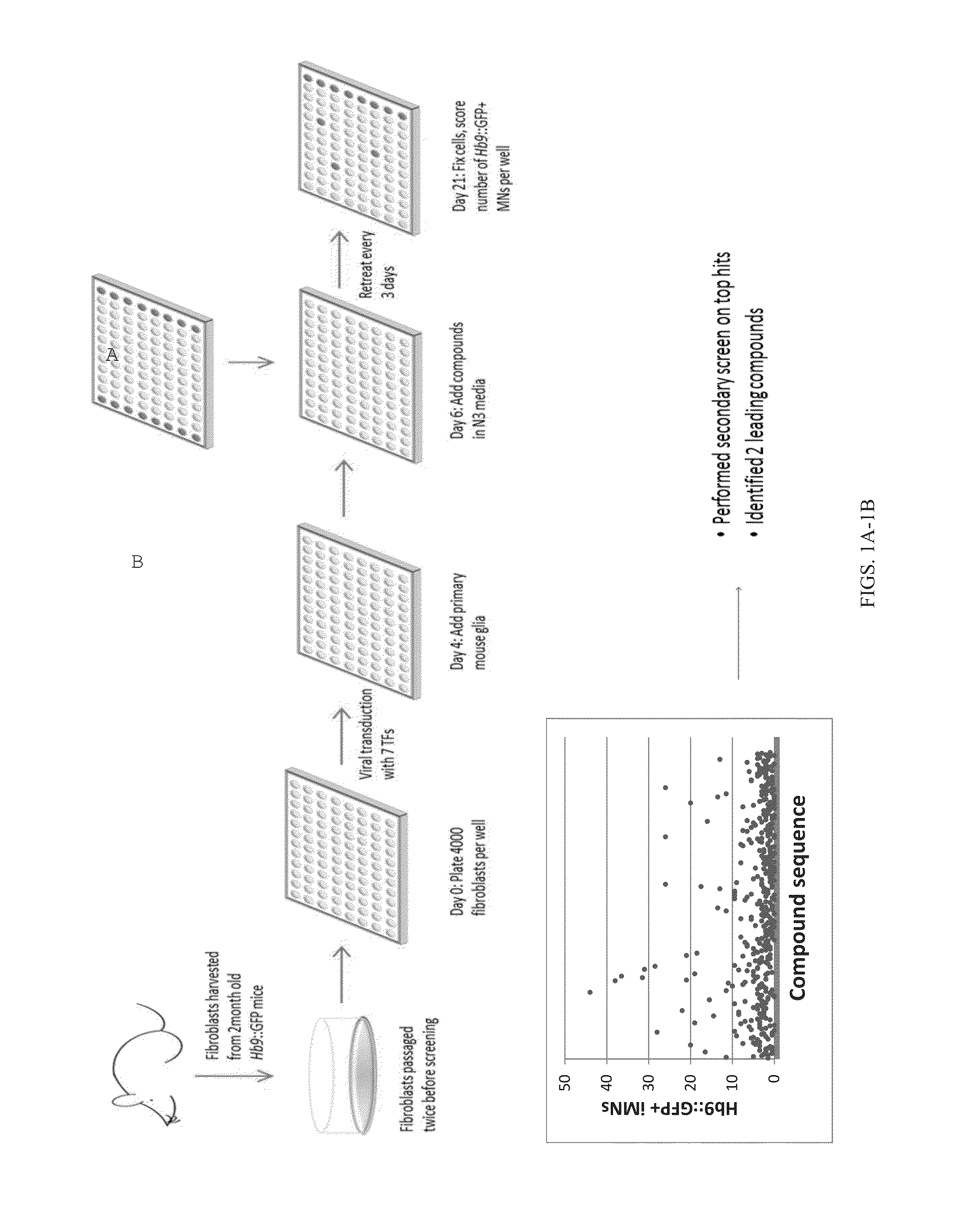
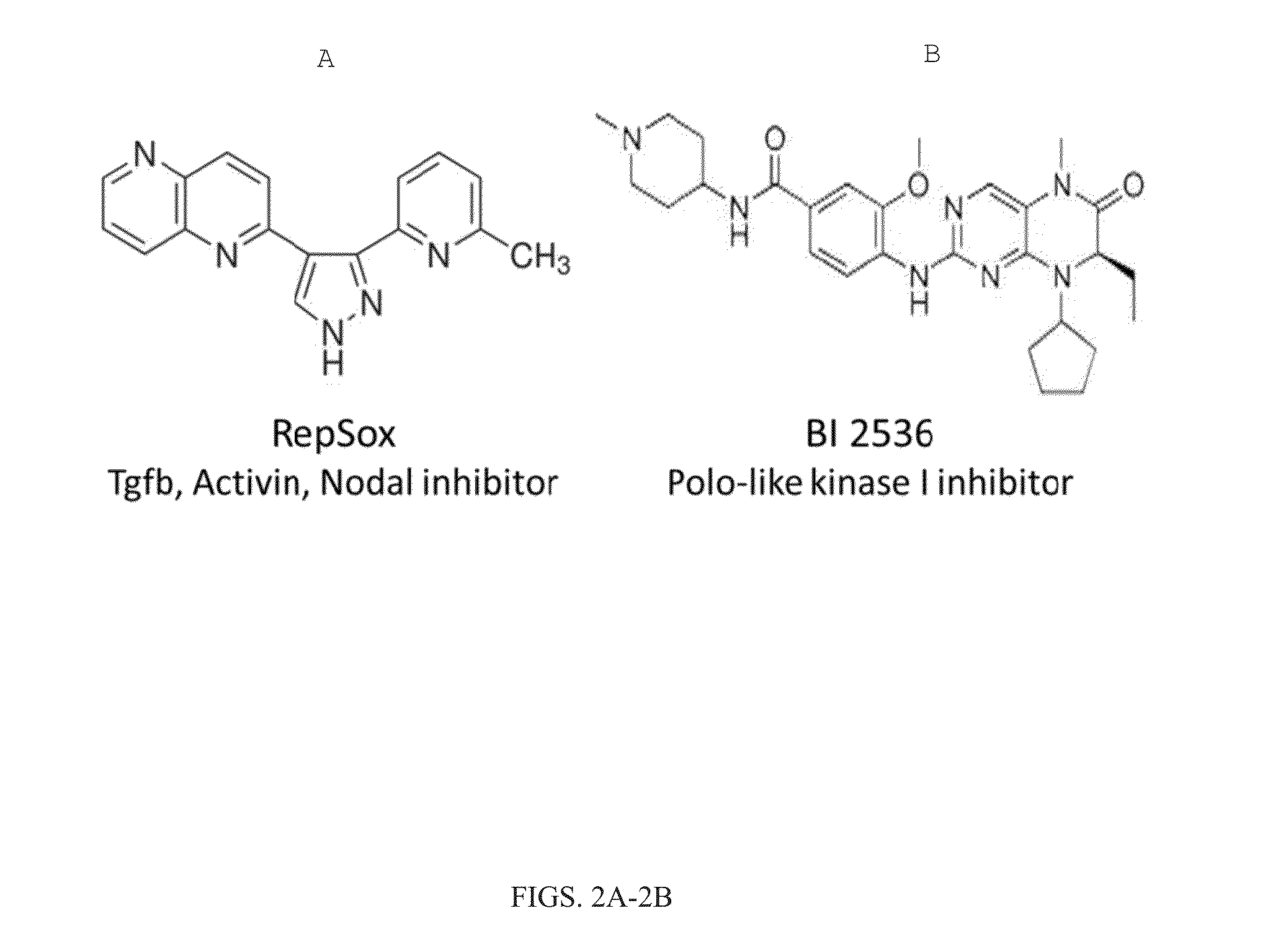
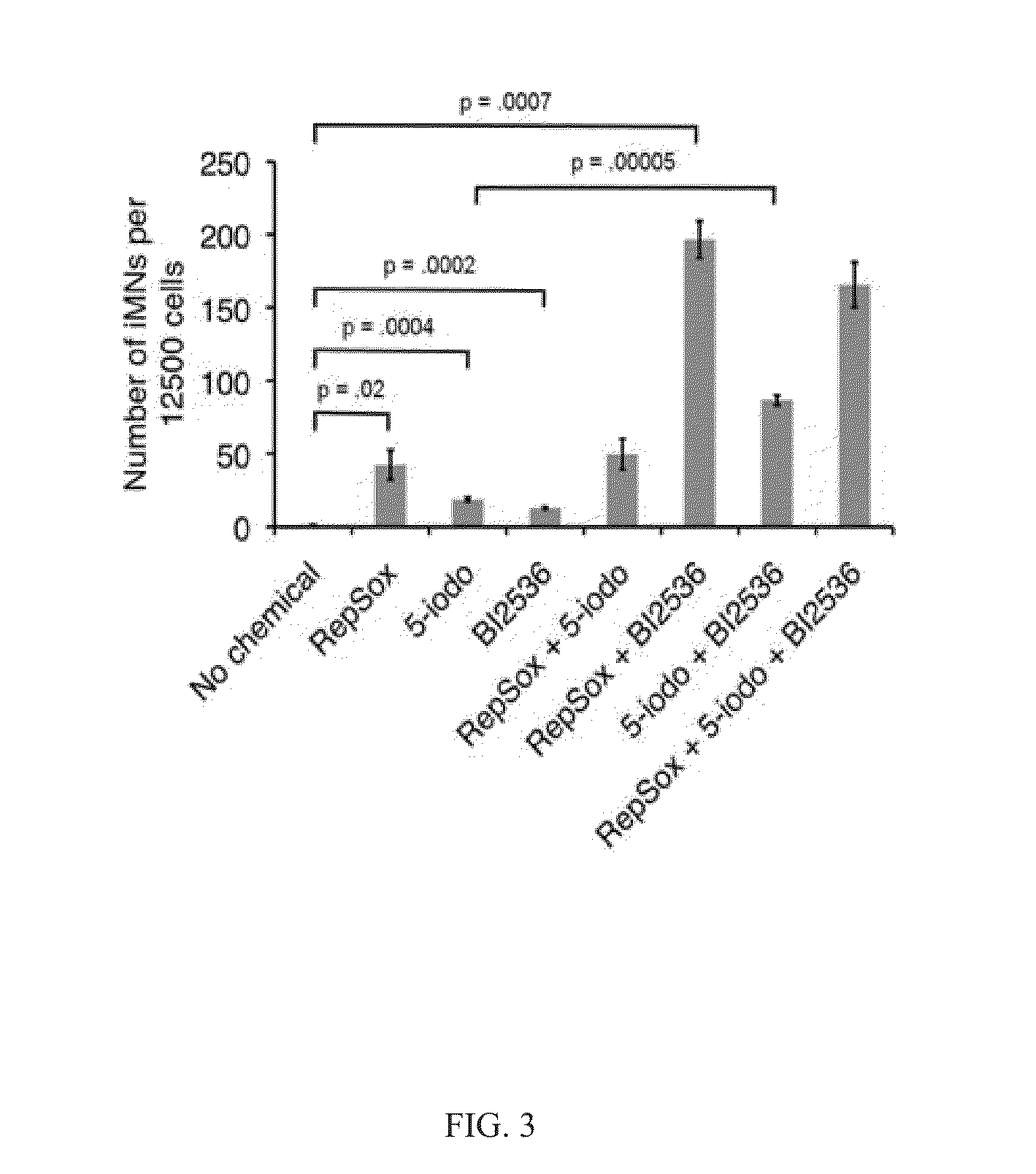
The present inventions relate to methods and compositions useful for improving the efficiency of inducing the generation of neurons from non-neuronal cell types, for example, by contacting the cell or cell culture medium with one or more agents which inhibit Activin and / or PLK1 signaling. Also disclosed are methods for promoting neuron survival, for example, by inhibiting Activin and / or PLK1 signaling, and methods for promoting the survival of intermediates in a cell differentiation pathway, for example, by inhibiting Activin and / or PLK1 signaling.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Methods and compositions for producing a neurosalutary effect in a subject
InactiveUS20050059594A1Conducive to survivalHormone peptidesOrganic active ingredientsNeuron survivalEndocrinology
Owner:BENOWITZ LARRY I
Pigment epithelium-derived factor: characterization, genomic organization and sequence of the PEDF gene
InactiveUS20060008900A1Senses disorderNervous disorderPIGMENT EPITHELIUM-DERIVED FACTORNeuron survival
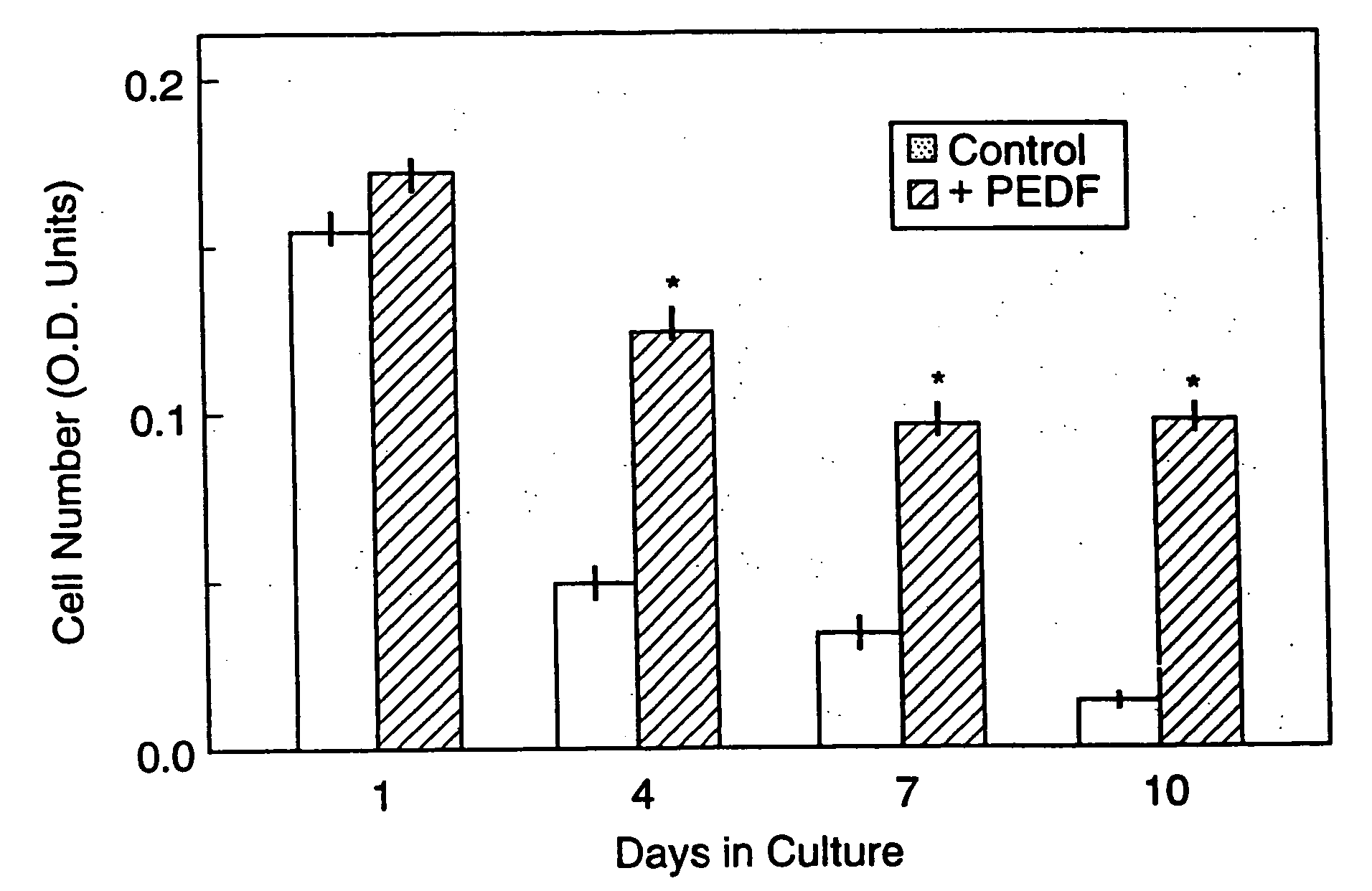
Nucleic acids encoding the neurotrophic protein known as pigment epithelium-derived factor (PEDF), a truncated version of PEDF referred to as rPEDF, and equivalent proteins, vectors comprising such nucleic acids, host cells into which such vectors have been introduced, recombinant methods for producing PEDF, rPEDF, and equivalent proteins, the rPEDF protein and equivalent proteins of rPEDF and PEDF-BP, -BX and BA, and the PEDF protein produced by recombinant methods. Effects and uses of these variants on 1) neuronal differentiation (neurotrophic effect) 2) neuron survival (neuronotrophic effect) and 3) glial inhibition (gliastatic effect) are described.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Devices containing DNA encoding neurotrophic agents and related compositions and methods
InactiveUS20080152689A1Efficient transferPromotes neuronal survivalPowder deliveryVirusesNervous systemAnti fibrotic
Devices useful in the delivery of DNA encoding neurotrophic agents, anti-fibrotic agents, and related compositions are disclosed herein for use in the treatment of central and / or peripheral nervous system injury. Methods of making and using the disclosed devices and DNA are also described. In various embodiments, the invention also discloses compositions and devices that may further include a targeting agent, such as a polypeptide that is reactive with an FGF receptor (e.g., bFGF), or another ligand that binds to cell surface receptors on neuronal cells, or a support cell. The invention also discloses methods of promoting neuronal survival and regeneration via transfection of an axon as it grows through a device or composition of the present invention, or via transfection of a repair cell.
Owner:TISSUE REPAIR CO +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com