Devices containing DNA encoding neurotrophic agents and related compositions and methods
a neurotrophic agent and dna technology, applied in the field of neurotrophic agent treatment, can solve the problems of preventing reconnection of neural pathways, affecting axonal regrowth following ns injury, and threatening neuronal survival, so as to promote neuronal survival, promote neuronal survival, and effectively transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
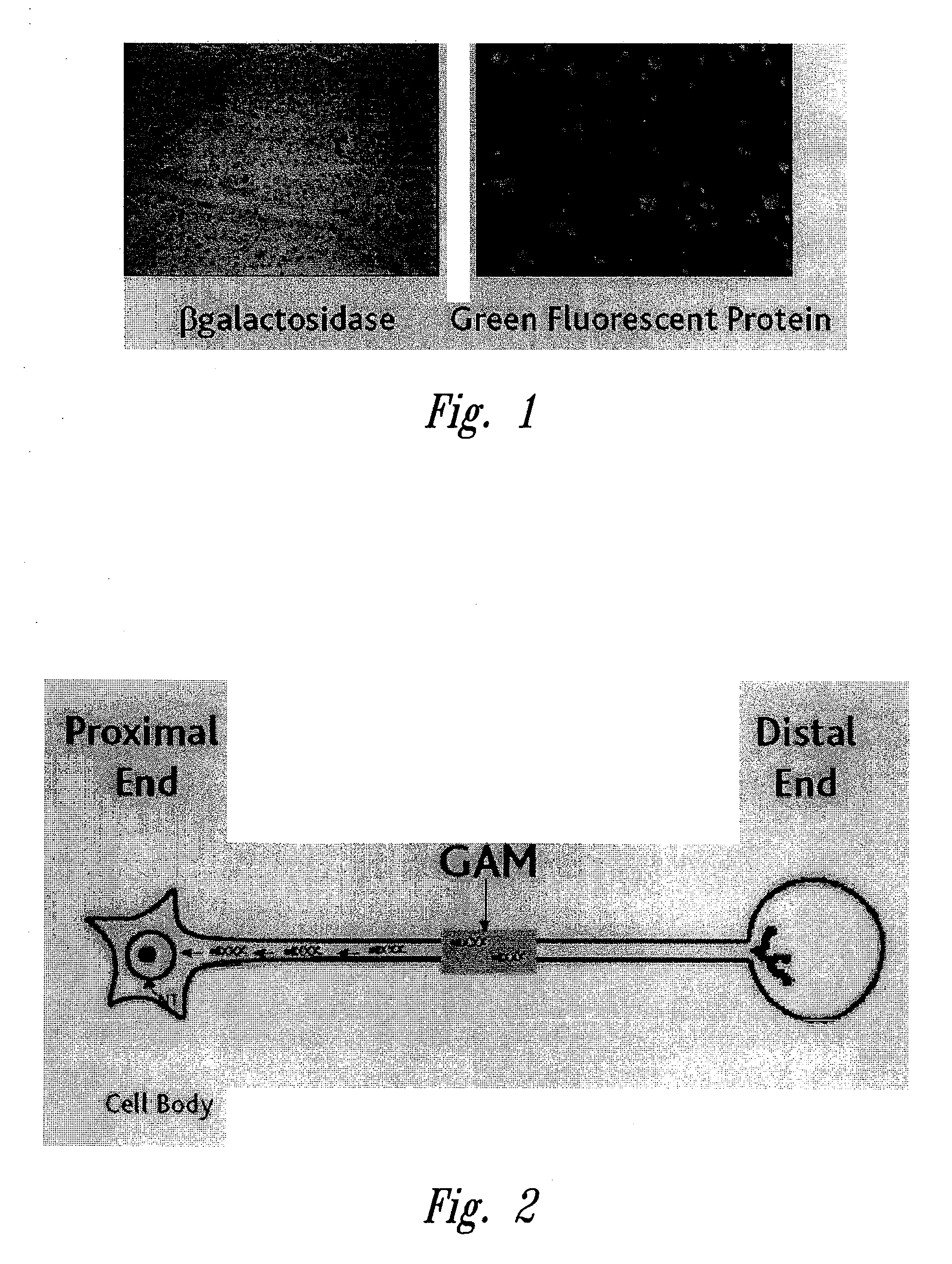
Preparation of Gene Activated Matrix Containing FGF2-Poly-L-Lysine Complexed with a Plasmid Encoding GFP Protein (GFP) Reporter Gene Under Promoter Regulation
[0276]Plasmid isolation, production of competent cells, transformation and manipulations using the M13 cloning vectors are performed as described (Sambrook et al., Molecular Cloning, a Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1989). DNA fragments are purified using the Geneclean II kit, purchased from Bio 101 (La Jolla, Calif.). Recombinant DNA constructs are sequenced using the Sequenase kit (version 2.0, United States Biochemical, Cleveland, Ohio) according to the manufacturer's instructions. Conjugation of FGF2 to poly-L-lysine K84 homopolymer to produce FGF2-K is as described by Sosnowski et al. (1996 J. Biol. Chem. 271:33647-33653). Preparation of FGF2-poly-L-lysine complexed with a plasmid encoding green fluorescent protein (GFP) under CMV promoter regulation is also essentially as...
example 2
Preparation of DNA Construct Containing the Neuronal GAP43 Promoter
[0280]Plasmid isolation, production of competent cells, transformation and manipulations using the M13 cloning vectors are performed as described (Sambrook et al., Molecular Cloning, a Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1989). DNA fragments are purified using the Geneclean II kit, purchased from Bio 101 (La Jolla, Calif.). Recombinant DNA constructs are sequenced using the Sequenase kit (version 2.0, United States Biochemical, Cleveland, Ohio) according to the manufacturer's instructions. DNA containing the human GAP43 promoter sequence (Genbank accession number X840768) is obtained as described in de Groen et al. (J. Mol. Neurosci. 6:109-119, 1995) and incorporated into plasmids in operative linkage with reporter gene encoding or neuronal therapeutic agent encoding sequences.
example 3
Delivery and Expression of Targeted GFP Gene in Lesioned Rat Optic Nerve Repair Model
[0281]In this example, targeted delivery of the GFP reporter gene to rat optic nerve neurons is conducted using a ligand as a molecular targeting agent in an in vivo model of neuronal regeneration.
[0282]Transgene expression of neuronal cells in vivo following experimentally induced axonal lesion is monitored in the rat optic nerve repair model. See, e.g., Logan et al., Meth. Neurosci. 21:3-19, 1994, which is hereby incorporated by reference in its entirety. Adult rats are anesthetized by intraperitoneal injection of physiological saline solution containing ketamine (40 mg / kg), acepromazine (1.2 mg / kg) and xylazine (8 mg / kg). The optic nerve is accessed intraorbitally by a dorsolateral approach and severed by transection using manual pressure applied with surgical forceps. (Berry et al., J. Neurocytol. 25:147-170, 1996). Care is taken to avoid damaging the central retinal artery or the optic nerve sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| inner diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com