Primer and probe for screening spinal muscular atrophy (SMA) genes and using method of primer and probe
A probe and gene technology, applied in the field of human spinal muscular atrophy (SMA) gene screening, can solve the problems of single cell detection, cumbersome operation steps, and poor repeatability of results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
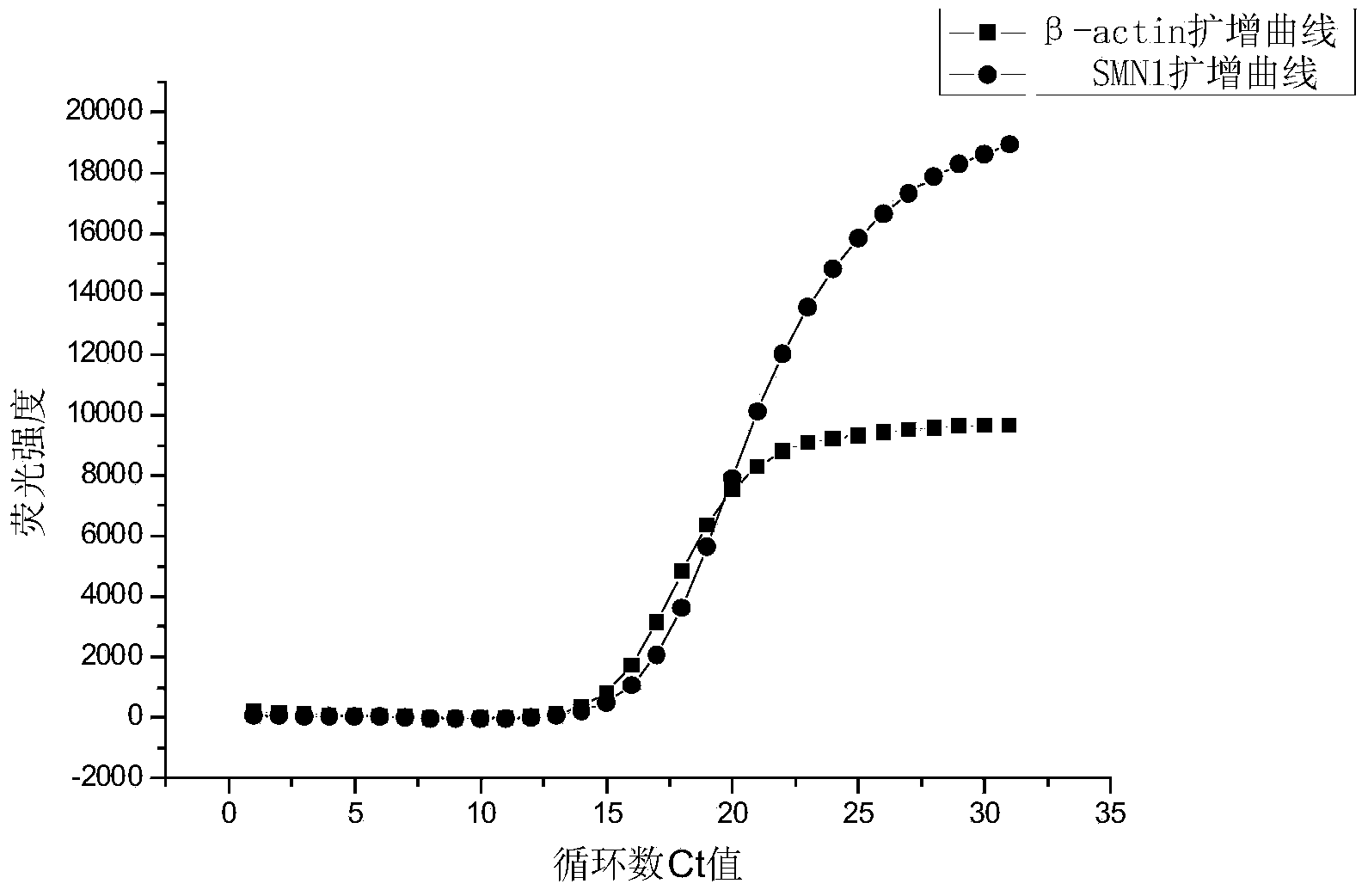
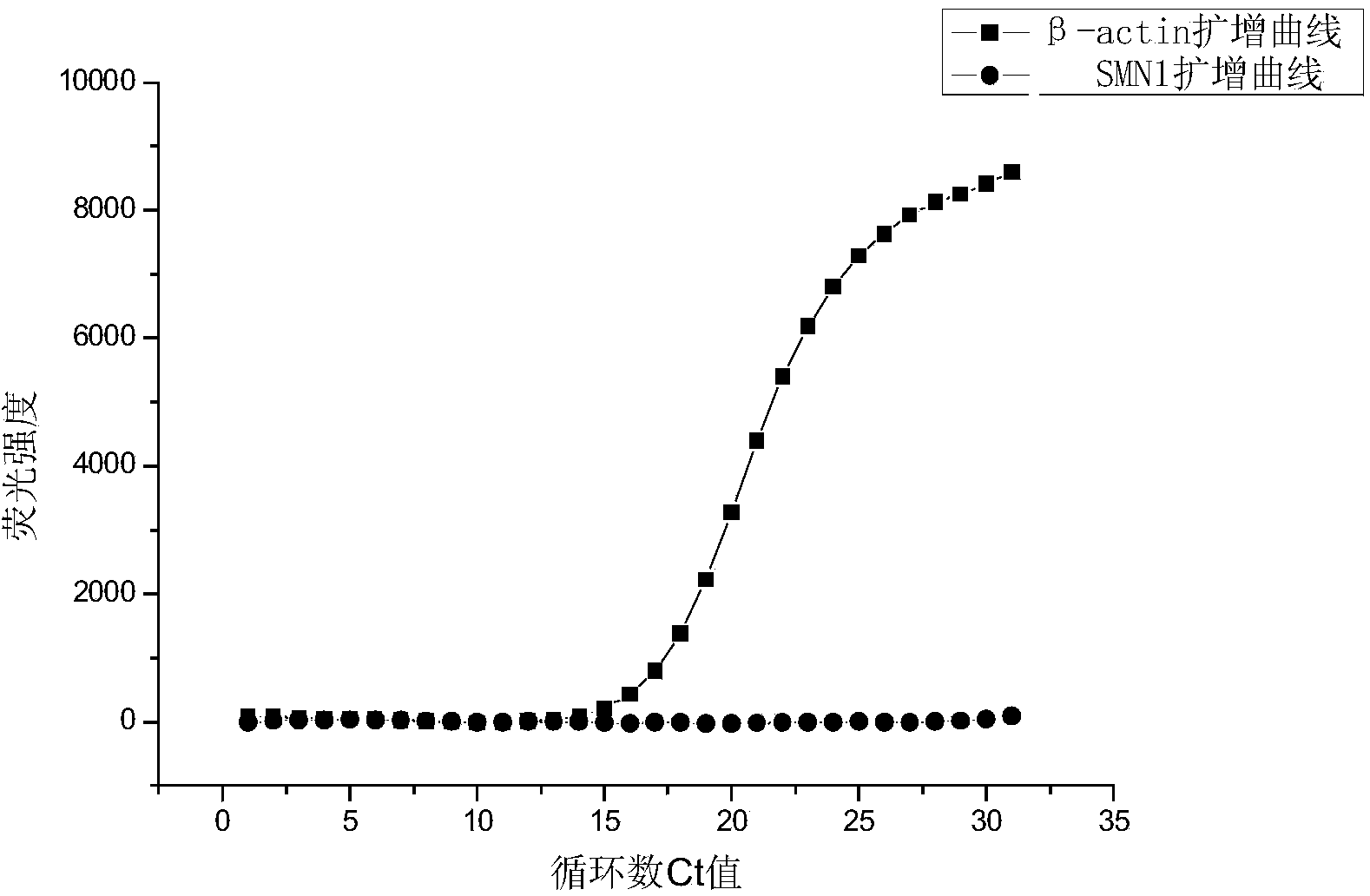
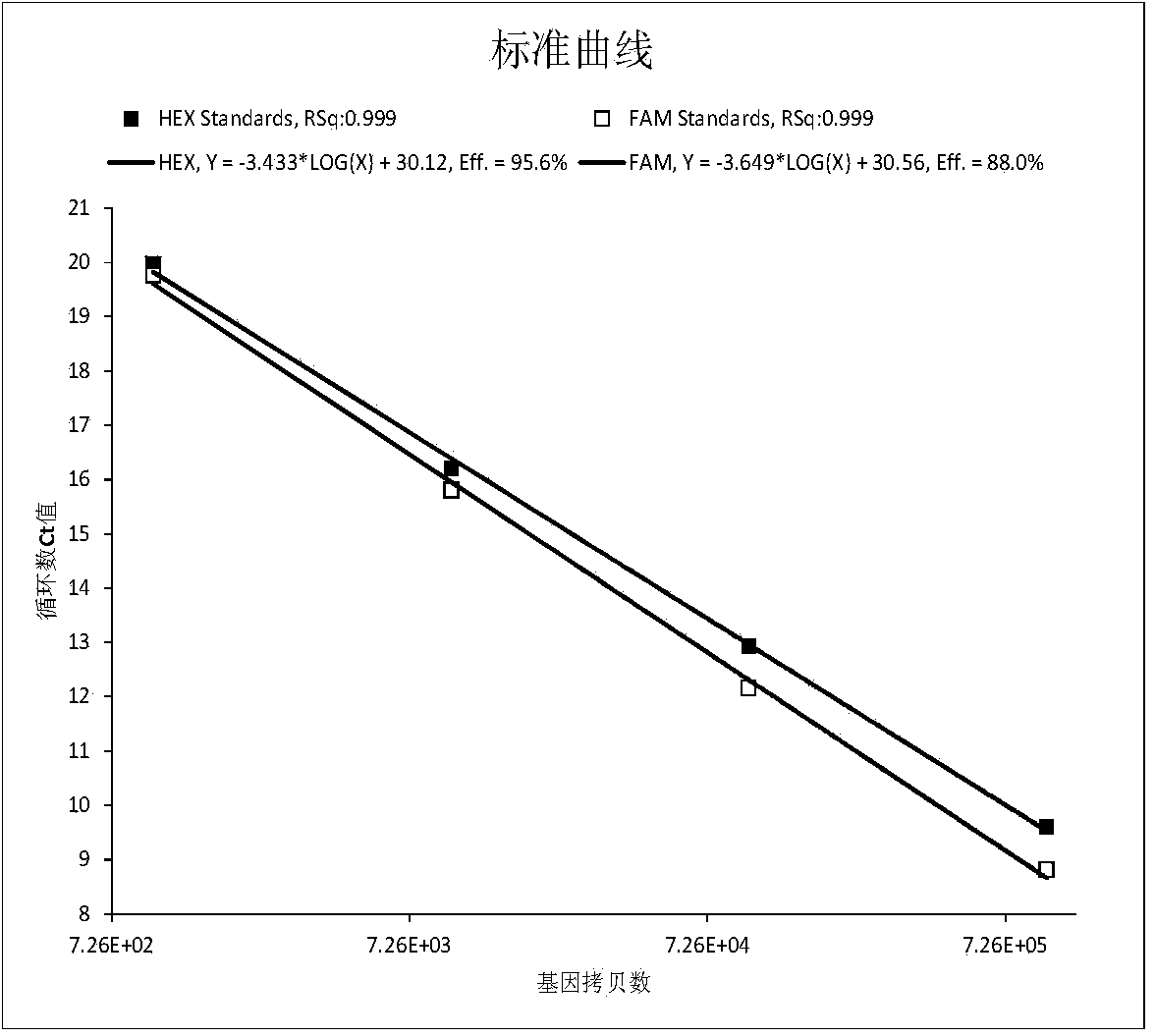
[0131] In this embodiment, the detection of the SMN1 gene is taken as an example to illustrate the method of single-tube fluorescent PCR detection of the sample to be tested.
[0132] The samples used in the experiment were the whole blood samples of 50 couples with no family history of SMA inheritance and the whole blood samples of 30 couples with family history of SMA inheritance.
[0133] The method for distinguishing normal people and carriers among 160 people using the above primers and probes for Survival Motor Neuron 1 (SMN1) screening includes the following steps:
[0134] (1) DNA extraction of samples:
[0135] 400 μL of whole blood samples were drawn from each person, and DNA was extracted using RBC’s MagCore Genomic DNA Whole Blood Kit (Cat. No: MGB400-04) according to the operating instructions of the kit; Adjust the extracted DNA to 10ng / μL with Tris-HCl solution (10mmol / L, pH8.0) as a template for PCR amplification;
[0136] (2) Fluorescent PCR amplification: ...
Embodiment 2
[0165] This embodiment takes the detection of SMN1 and SMN2 genes as an example to illustrate the method of detecting whether the fetus is a normal person or a patient by single-tube fluorescent PCR.
[0166] The samples used in the experiment were the amniotic fluid samples of 50 pregnant women whose husband and wife were both carriers.
[0167] Use the above primers and probes for survival motor neuron 1 (SMN1) screening and primers and probes for survival motor neuron 2 (SMN2) screening to determine whether 50 fetuses are normal or patients The method includes the following steps:
[0168] (1) DNA extraction of samples:
[0169] 400 μL amniotic fluid samples were drawn from each pregnant woman, and then the amniotic fluid samples were first passed through Biological Industries’ BIOAMF-2Complete Medium For Human Amniotic Fluid and Chorionic Villi Samples medium (Cat.No: 01-194-1), and operated according to the medium Note that after cell culture, take no more than 1×10 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com