1,2,3,4-tetrahydroisoquinoline derivatives having effects of preventing and treating degenerative and inflammatory diseases
A degenerative disease, tetrahydroisoquinoline technology, applied in bone diseases, organic active ingredients, nervous system diseases, etc., can solve the problem that no effective method for neurodegenerative diseases has been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
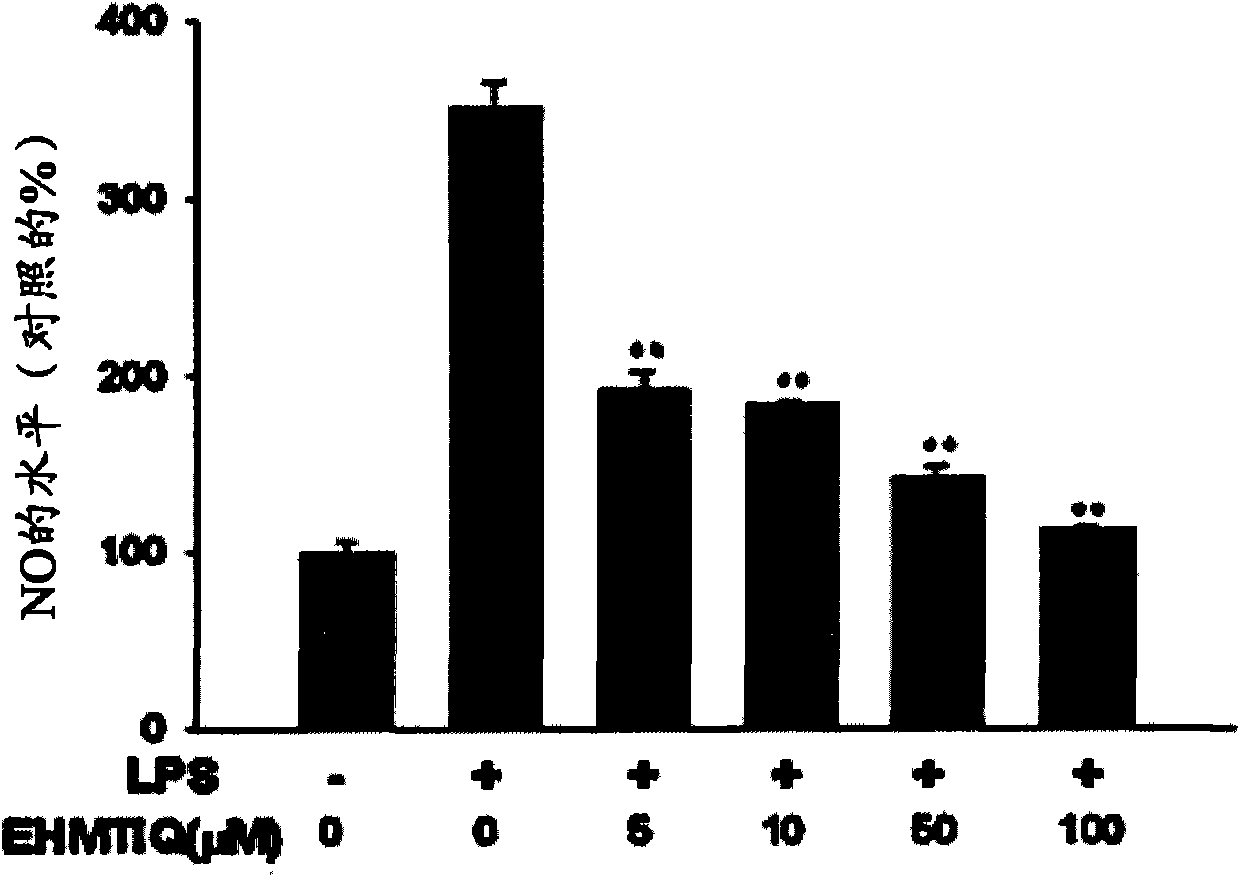
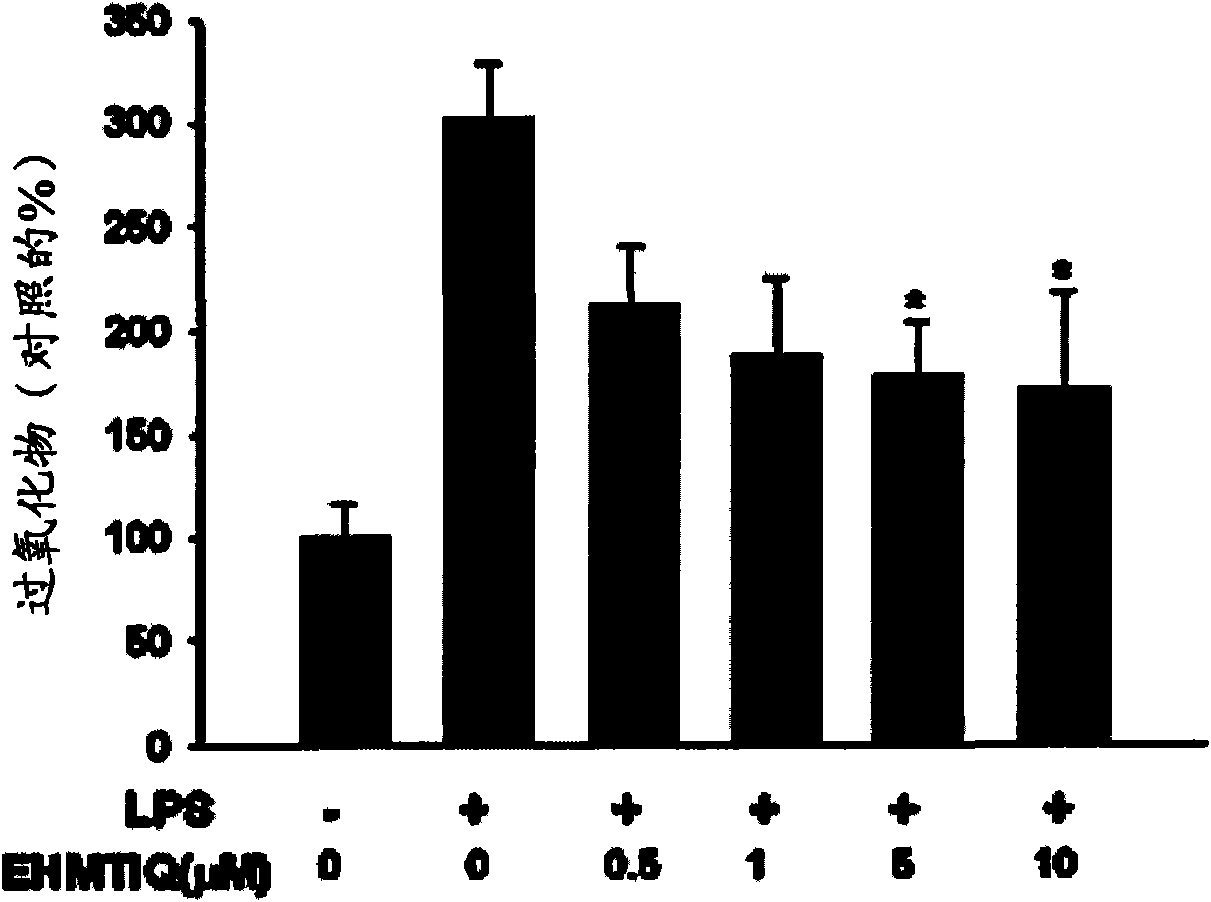
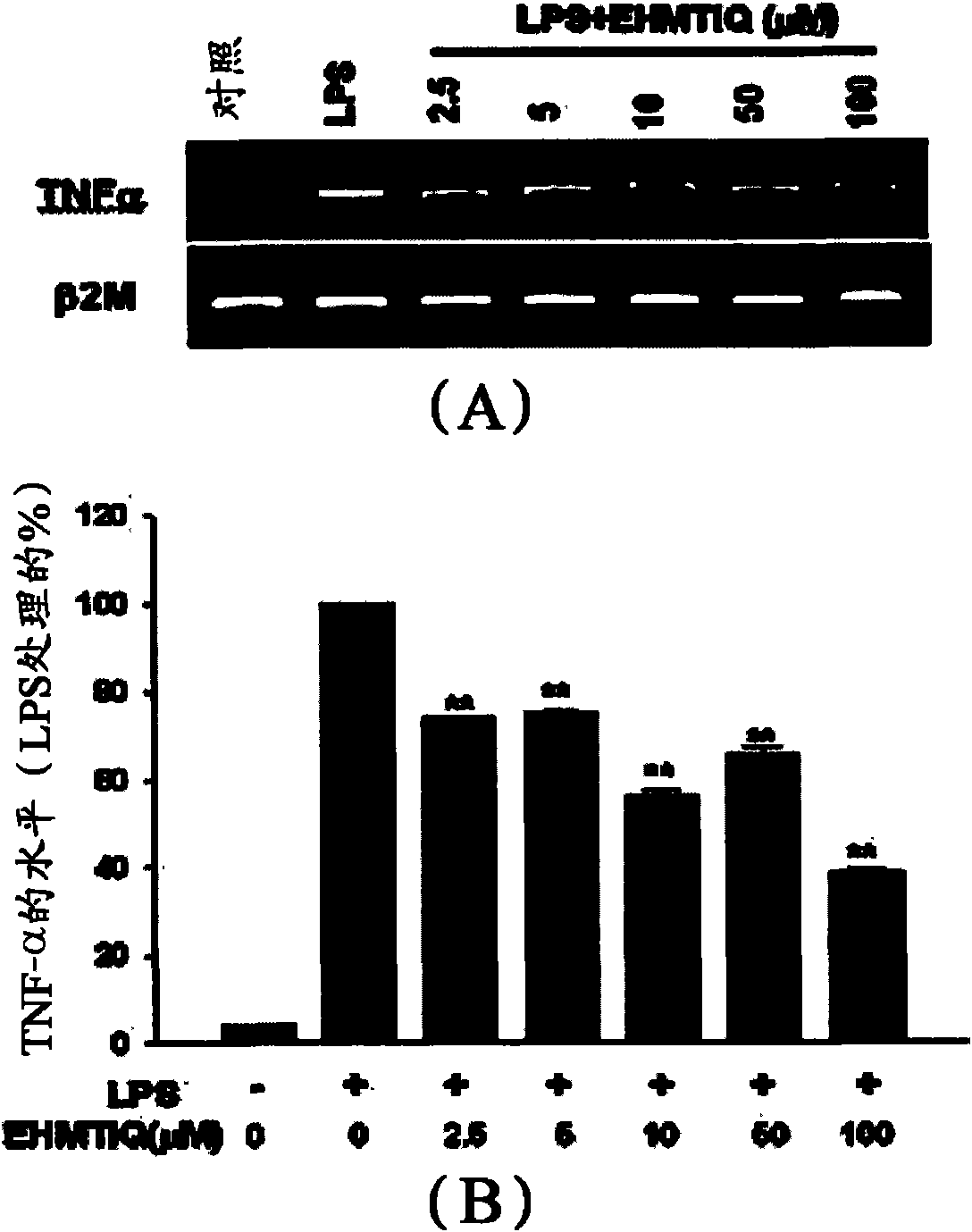
Image
Examples
Embodiment 1
[0070] Example 1: 2-acetyl-7-hydroxy-6-methoxy substituted with methyl or phenyl at the C1 position -Synthesis of 1,2,3,4-tetrahydroisoquinoline (AHMTIQ) derivatives and their structural analysis
[0071] [Chemical Equation 1]
[0072]
[0073] Preparation and Analysis of AHMTIQ(5a)
[0074] Acetaldehyde (15.7mmol, 692mg) was reacted with 3-O-methyldopamine hydrochloride (1.96mmol, 400mg) dissolved in 1M HCl solution (10ml) in a pressure tube at 100°C for 24 hours. The reaction tube was allowed to cool and the mixture was neutralized with sodium bicarbonate. Water and remaining solvent were removed under reduced pressure, and the resultant was dried in vacuo. Methanol was added to the residual precipitate on the filter and crude compound 4a was extracted by short column chromatography. Crude compound 4a was crystallized to obtain the hydrochloride salt (250 mg, 55%) as a white powder [ 1 HNMR (DMSO-d 6 , 400MHz) 89.93(br s, 1H), 9.40(br s, 1H), 9.06(s, 1H), 6.72(s,...
Embodiment 2
[0080] Example 2: AHMTIQ derivatives (6, 7a-h, 8a-h and 9a-g) synthetic methods and their structural analysis
[0081] [Chemical Equation 2]
[0082]
[0083] ①N-(2-(4-hydroxy-3-O-methylphenyl) ethyl)-tert-butyl carbonate (6) synthetic method and analysis analysis
[0084] Method a) tert-butoxycarbonyl anhydride (7.63mmol, 1.67g) and triethylamine (19.5mmol, 1.93g) and compound 3 (6.35mmol, 1.30g) were added into chloroform (20ml). The mixture was stirred at RT for 24 hours, to which was added aluminum chloride solution. The mixture was extracted with dichloromethane solvent, and the organic layer was washed twice with water. White crystalline compound (1.32 g, 59%) was obtained by column chromatography and recrystallization [ 1 H NMR (CDCl 3 , 200MHz) δ6.83(d, J=8.4Hz, 1H), 6.63-6.67(m, 2H), 5.83(s, 1H), 6.45(br s, 1H), 3.84(s, 3H), 3.35( q, J=6.6Hz, 2H), 2.70(t, J=7.0Hz, 2H), 1.43(s, 9H); 13 C NMR (CDCl 3 , 50MHz) δ155.9, 146.5, 144.1, 130.6, 121.2, 114.4, ...
Embodiment 3
[0141] Example 3: 7-Hydroxy-6-methoxy-1,2,3,4-tetrahydroiso Synthesis of Quinoline (HMTIQ) Derivatives (11a-e and 12a-f) and Their Structural Analysis
[0142] [Chemical Equation 3]
[0143]
[0144] ①Preparation and analysis of HMTIQ derivatives (11a-e) substituted with amides at the N2 position
[0145] Preparation a) and b): Compound 10 (1.0 mmol or 2.0 mmol) was dissolved in dichloromethane solvent (10-15 ml), and alkyl acid chlorides (propionic anhydride, butyryl chloride, cyclohexanoyl chloride, iso butyryl chloride or 3-methylbutyryl chloride). Triethylamine (3.0 mmol or 6.0 mmol) was added slowly and the mixture was stirred at RT for about 1 hour. The reaction was quenched with water, and the organic layer was washed with water. The solvent was removed under reduced pressure. The obtained compound was dissolved in methanol (10-20 ml), and calcium carbonate (3.0 mmol or 6.0 mmol) was added thereto, and then the mixture was refluxed for about 2-3 hours. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com