Combination therapy involving diaryl macrocyclic compounds
A compound, immunotherapy technology, used in refrigeration and liquefaction, medical preparations containing active ingredients, heating methods, etc., can solve problems that have not yet caused successful treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0600] In some embodiments, the methods described herein relate to cancer treatment comprising administering to a patient in need of treatment a therapeutically effective amount of one or more compounds that inhibit FAK, SRC and / or JAK2, and trametinib. In some embodiments, the methods described herein relate to cancer treatment comprising administering to a patient in need of treatment a therapeutically effective amount of a compound that inhibits FAK, SRC and JAK2, and trametinib. It is to be understood that an inhibitor is one that reduces or inhibits the activity of another substance, such as a cell surface receptor (ie, receptor tyrosine kinase) or kinase (ie, non-receptor tyrosine kinase), or the transcription and / or transcription of a gene. or any substance translated. It will be appreciated that a "compound that inhibits FAK, SRC and JAK2" is a compound that has affinity for all three biological targets FAK, SRC and JAK2.
[0601] It has been discovered that certain o...
example 1
[0668] Example 1: NSCLC cell viability assay
[0669] One to two thousand cells per well were seeded in 96 or 384 well white dishes and then treated with the indicated compounds for 72-120 hours (37°C, 5% CO 2 ). Cell proliferation was measured using the CellTiter-Glo luciferase-based ATP detection assay (Promega) following the manufacturer's protocol. IC was performed using GraphPad Prism software (GraphPad, Inc., San Diego, CA) 50 value determination.
[0670] exist Figure 1a Cells displaying a MEK1 / 2 inhibitor (trametinib), Compound 1, and a combination of a MEK1 / 2 inhibitor (trametinib) and Compound 1 (1 μM) in a mutant KRAS NSCLC cell line are shown in -1x Vitality % results. IC 50 The values are summarized in Table 1. Although Calu-1, COR-L23, HCC1588, LCLC-97TM1, LU2512, NCI-H1155, NCI-H1373, NCI-H1573, SK-LU-1 and SW1573 NSCLC cell lines endogenously express KRAS mutations, MEK inhibitors Trametinib exhibited weak to undetectable inhibition of cell proliferat...
example 2
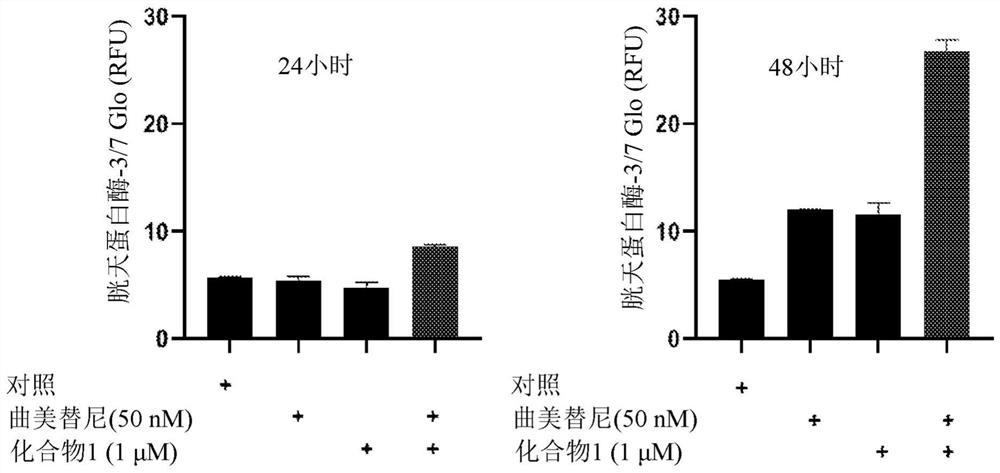
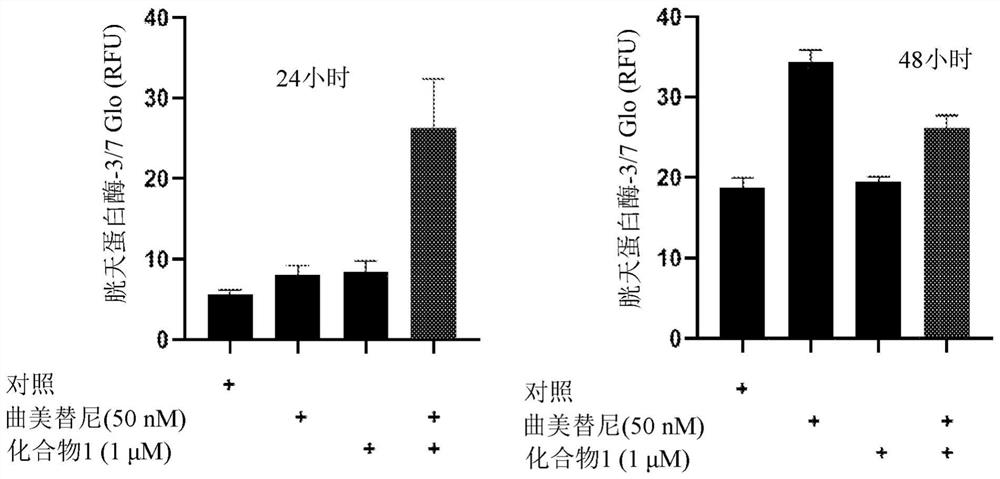
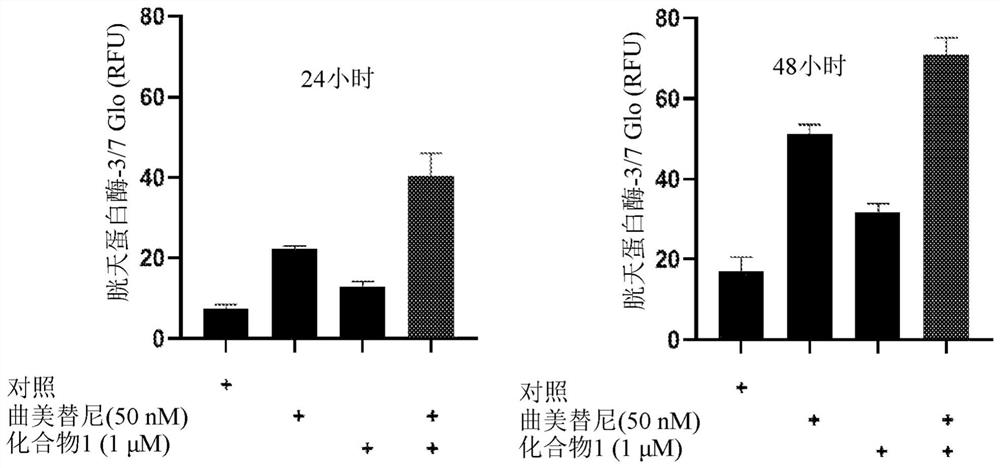
[0674] Example 2. Apoptosis analysis
[0675] Two thousand cells per well were seeded in 384-well white dishes and then treated with compounds for 24 or 48 hours (37°C, 5% CO 2 ). use The 3 / 7 Assay (Promega) followed the manufacturer's protocol to measure cellular caspase-3 / 7 activity (a major marker of apoptosis). exist Figures 1a-1d Shown in NSCLC cell lines with KRAS G12C mutations (H358, H2122), KRAS Q61H mutations (Calu-6), and KRAS G12V mutations (H441), in NSCLC cell lines with trametinib (50 nM), compound 1 ( 1 μM) and trametinib (50 nM) in combination with Compound 1 (1 μM) for 24 and 48 hours of treatment, results of increased caspase-3 / 7 activity. Compound 1 alone caused a modest increase in caspase-3 / 7 activity in NCI-H358, NCI-H2122 NSCLC cell lines after 48 hours of treatment ( Figure 1a , 1c ). Trametinib alone increased caspase-3 / 7 activity in H358, Calu-6, H2122 NSCLC cell lines after 48 hours of treatment ( Figures 1a-1c ). Compared to trametinib ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com