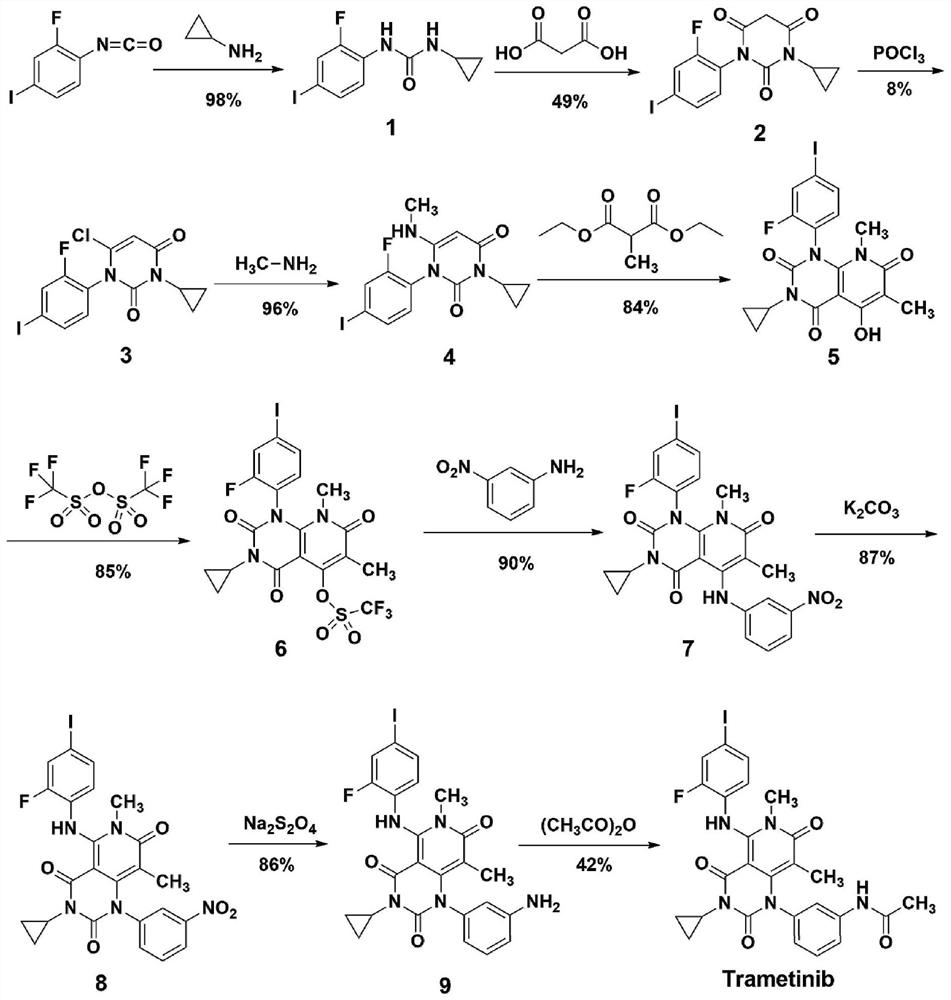
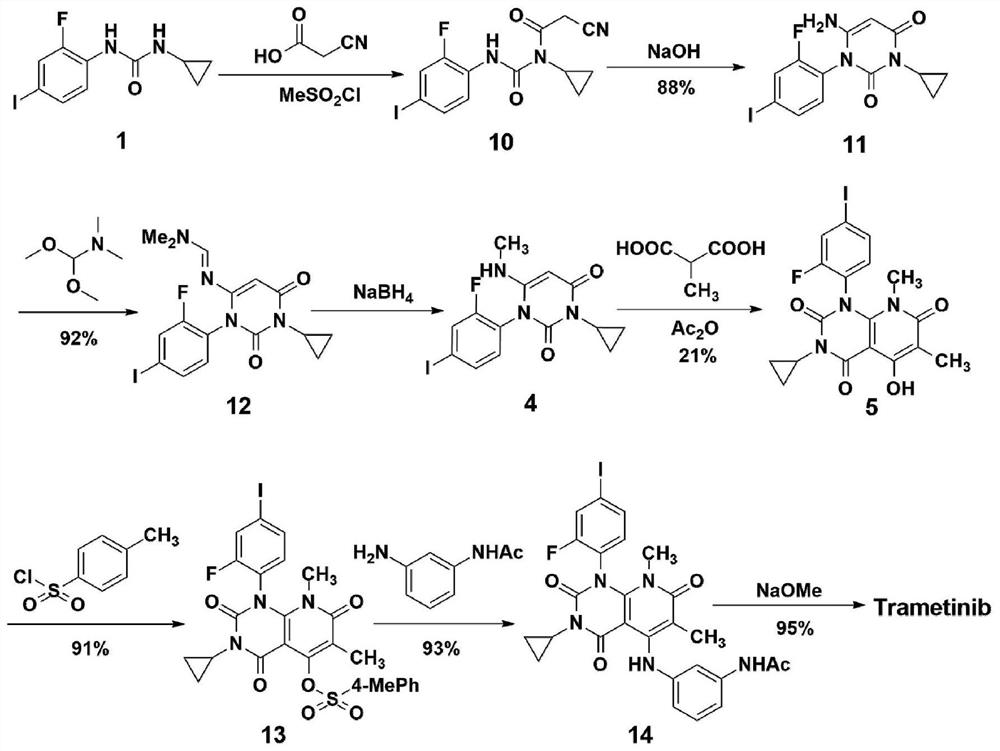
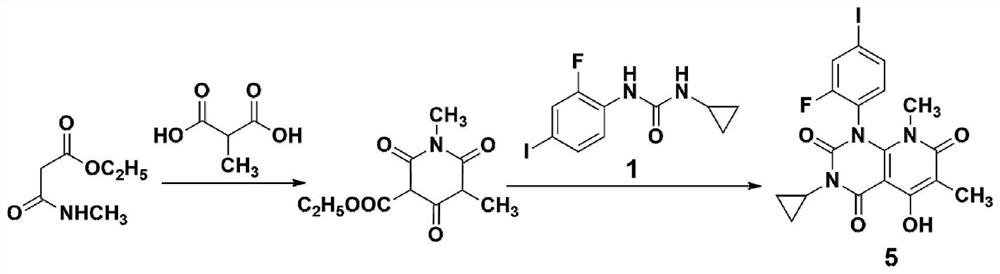
A method for synthesizing trametinib key intermediate
A technology for trametinib and intermediates, which is applied in the field of synthesizing key intermediates of trametinib, can solve the problems of many steps, low yield of one step, low yield of key steps, etc., and achieves the effect of short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] according to image 3 The shown roadmap prepares the key intermediate of synthetic trametinib:
[0034] S1: At room temperature, oxalyl chloride (13.4mL, 153mmol) was slowly added dropwise to a suspension of methylmalonic acid (9.0g, 76.2mmol) and DMF (2 drops) in dichloromethane (500mL), and reacted at room temperature for 24h , the solution was homogeneous, and slowly added dropwise to a solution of monoethyl malonate (9.2g, 63.4mmol) in dry toluene (100mL). NaOH solution (1mol / L, 100mL), separate the layers, take the solution layer, continue to add NaOH solution (1mol / L, 100mL) to the organic layer, combine the solution layers, wash with dichloromethane (200mL), adjust the pH to 1 with concentrated hydrochloric acid , ethyl acetate (50mL once, 3 times in total) extracted the aqueous layer, combined the ethyl acetate phases, dried, filtered, and concentrated to obtain a crude product, which was directly put into the next step without purification;
[0035] S2: To a ...
Embodiment 2
[0037]S1: At room temperature, slowly add oxalyl chloride (13.4mL, 153mmol) dropwise to a suspension of methylmalonic acid (9.0g, 76.2mmol) and DMF (2 drops) in dichloromethane (450mL), and react at room temperature for 20h , the solution was homogeneous, and was slowly added dropwise to a solution of monoethyl malonate (8.13g, 56mmol) in dry toluene (82mL). solution (0.8mol / L, 82mL), separate the layers, take the solution layer, continue to add NaOH solution (0.8mol / L, 82mL) to the organic layer, combine the solution layers, wash with dichloromethane (164mL), and adjust the pH to 1. Extract the aqueous layer with ethyl acetate (50 mL once, 3 times in total), combine the ethyl acetate phases, dry, filter, and concentrate to obtain a crude product, which is directly put into the next step without purification;
[0038] S2: To a solution of N-(2-fluoro-4-iodophenyl)-N'-cyclopropylurea (17.9g, 56mmol) in THF (280mL), add sodium ethylate (11.4 g, 168mmol), warmed to room temperat...
Embodiment 3
[0040] S1: Slowly add oxalyl chloride (13.4mL, 153mmol) dropwise to a suspension of methylmalonic acid (9.0g, 76.2mmol) and DMF (2 drops) in dichloromethane (540mL) at room temperature, and react at room temperature for 36h , the solution was homogeneous, slowly added dropwise to a solution of monoethyl malonate (11.05g, 76.2mmol) in dry toluene (132mL), after the addition was completed, the reaction was continued at 50°C for 20h, NaOH solution (1.2mol / L, 132mL), separate the layers, take the solution layer, continue to add NaOH solution (0.8mol / L, 82mL) to the organic layer, combine the solution layers, wash with dichloromethane (264mL), adjust the pH with concentrated hydrochloric acid To 1, extract the aqueous layer with ethyl acetate (50 mL once, 3 times in total), combine the ethyl acetate phases, dry, filter, and concentrate to obtain a crude product, which is directly put into the next step without purification;
[0041] S2: To a solution of N-(2-fluoro-4-iodophenyl)-N'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com