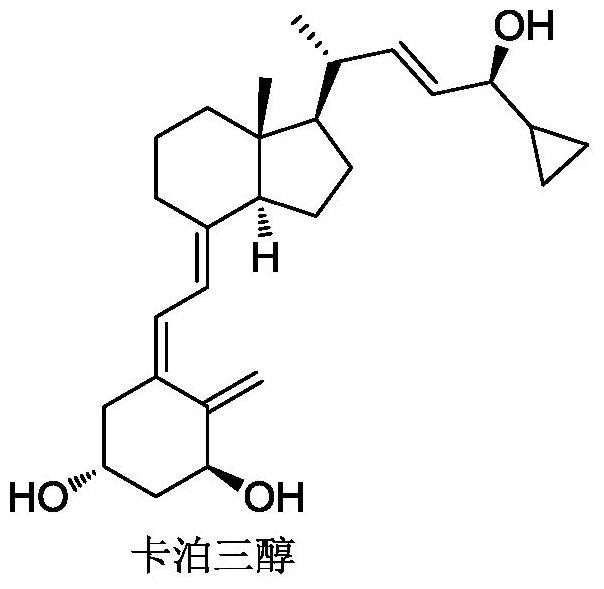
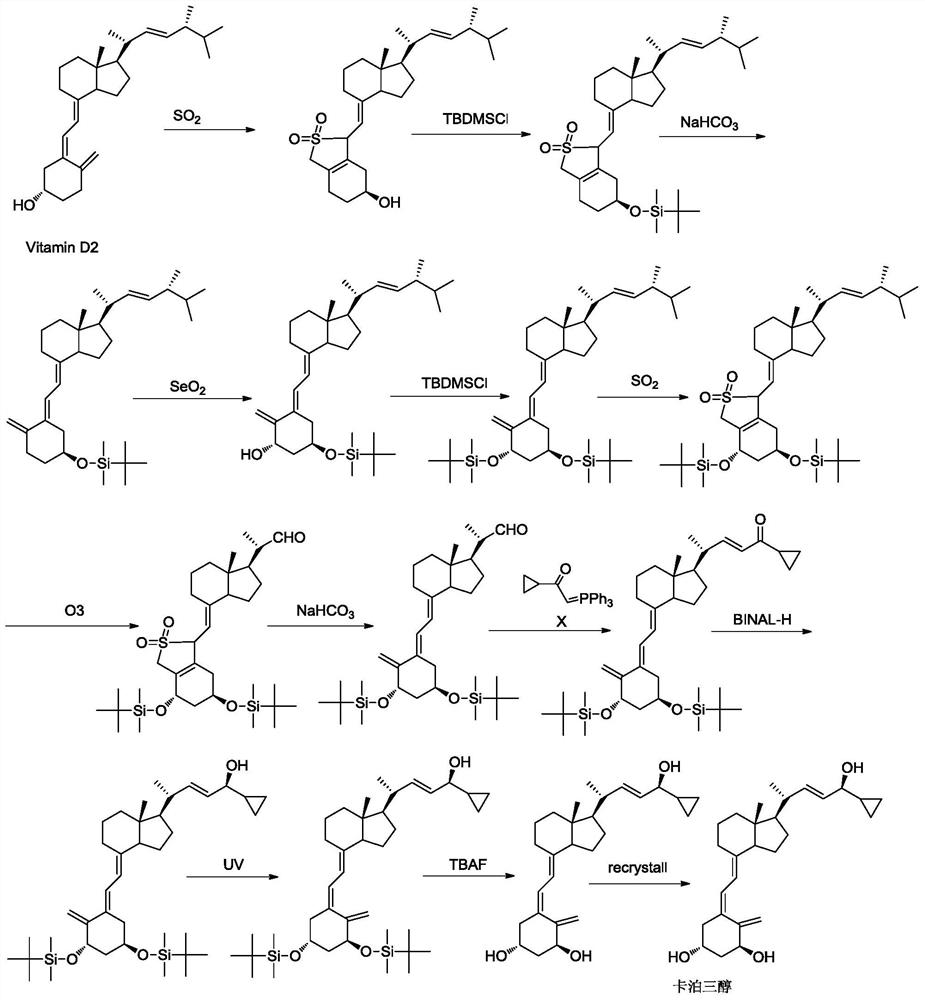
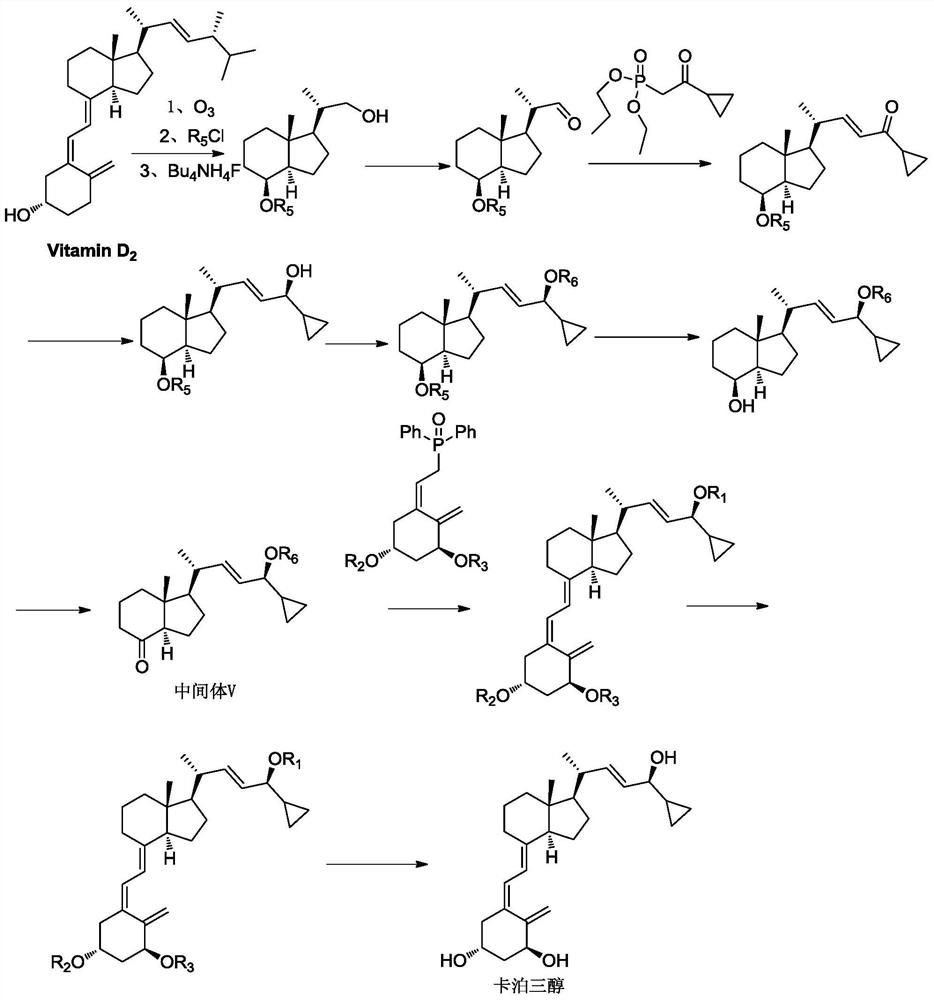
a preparation of vitamin d 3 Methods for Analog Intermediates
A technology of intermediates and analogs, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low selectivity of chiral reduction, difficulty in purification, long reaction steps, etc., to achieve industrial scale production, overcome long reaction time, unit The effect of less operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 intermediate IV
[0044]Under nitrogen protection, compound II (26.9g, 129.3mmol) was added to a 1000ml three-necked flask, raw material III (45.9g, 194.0mmol), tetrahydrofuran (300ml) was added, stirred evenly, cooled to -50°C, and the reaction was stirred for 2-3 Hours, after HPLC detects that the reaction is complete, the reaction solution is poured into ice water, stirred for 0.5-1.0 hours, the solution is extracted with n-hexane (200ml×3), washed with methanol:water=3:1 (50ml×3), and depressurized The solvent was distilled off to obtain 28.0 g of oil, with a yield of 78.4% and an HPLC purity of 96.7%.
[0045] The HPLC instrument and detection conditions are as follows:
[0046] Chromatographic column: Agilent ZORBAX SIL, 250×4.6mm, 5μm;
[0047] Mobile phase: n-hexane: isopropanol = 98:2, detection wavelength: 200nm;
[0048] Column temperature: 25°C, injection volume: 20μl, flow rate: 1.0ml / min.
Embodiment 2
[0049] Example 2 Vitamin D 3 Preparation of analog intermediate V
[0050] Add compound IV (52g, 188mmol), catalyst imidazole (23g, 338mmol) and 1000mL dichloromethane into a 250ml three-necked flask. After stirring evenly, add tert-butyldimethylsilyl chloride (42.1g, 280mmol) in three batches, After the addition is complete, react at room temperature for 2-2.5 hours. After the reaction is detected by TLC (developing agent n-hexane:ethyl acetate=10:1, Rf of IV=0.4, Rf of V=0.6), add 100ml of water to wash , liquid separation, the organic phase was washed with 100ml of water × 2, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure until no fraction flowed out to obtain oily vitamin D 3 The analog intermediate V was 62.5g, the yield was 85.1%, and the HPLC purity was 95.9%.
[0051] The HPLC instrument and detection conditions are as follows:
[0052] Chromatographic column: Agilent ZORBAX SIL, 250×4.6mm, 5μm; ...
Embodiment 3
[0055] Example 3 Vitamin D 3 Preparation of analog intermediate V
[0056] The compound represented by formula I in this embodiment can be obtained through commercial channels, and can also be obtained from vitamin D 2 Obtained by ozonation reduction reaction. Vitamin D 2 For the preparation of the compound represented by formula I by ozonation, reference can be made to document US20130006003.
[0057] (1) with vitamin D 2 Preparation of compound I
[0058] 400g Vitamin D 2 Dissolved in 4L of dichloromethane and 12L of methanol, the solution was cooled to -70°C, passed through ozone until the solution turned blue, the obtained ozonide was reduced with 308g of sodium borohydride, then washed with saturated brine, the organic layer was concentrated, and then passed through the column, Concentrate and dry to obtain 148g of compound I with a yield of 68%.
[0059] (2) Prepare compound II with compound I
[0060] Add compound I (26.1g, 123.1mmol) and dichloromethane (300ml)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com