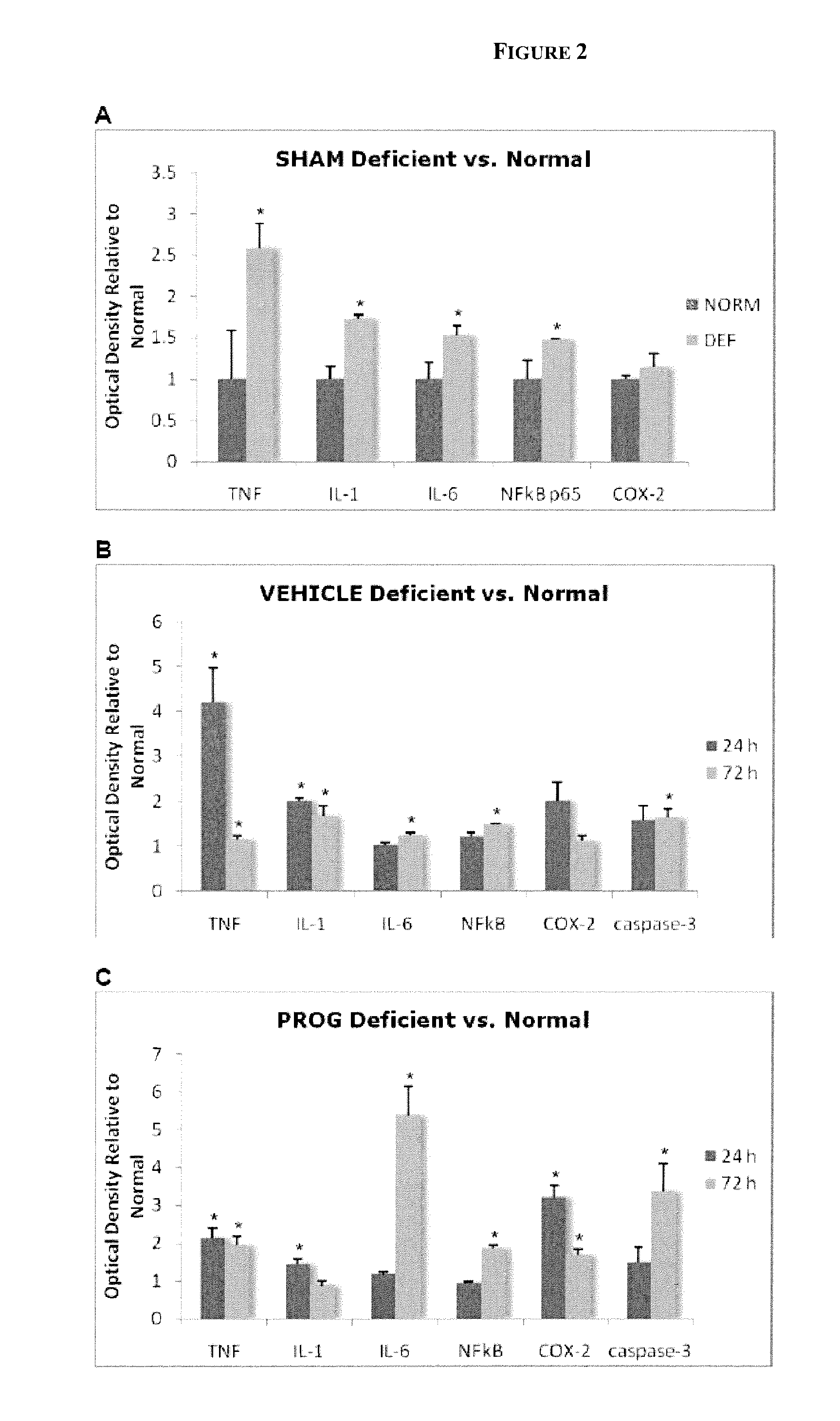
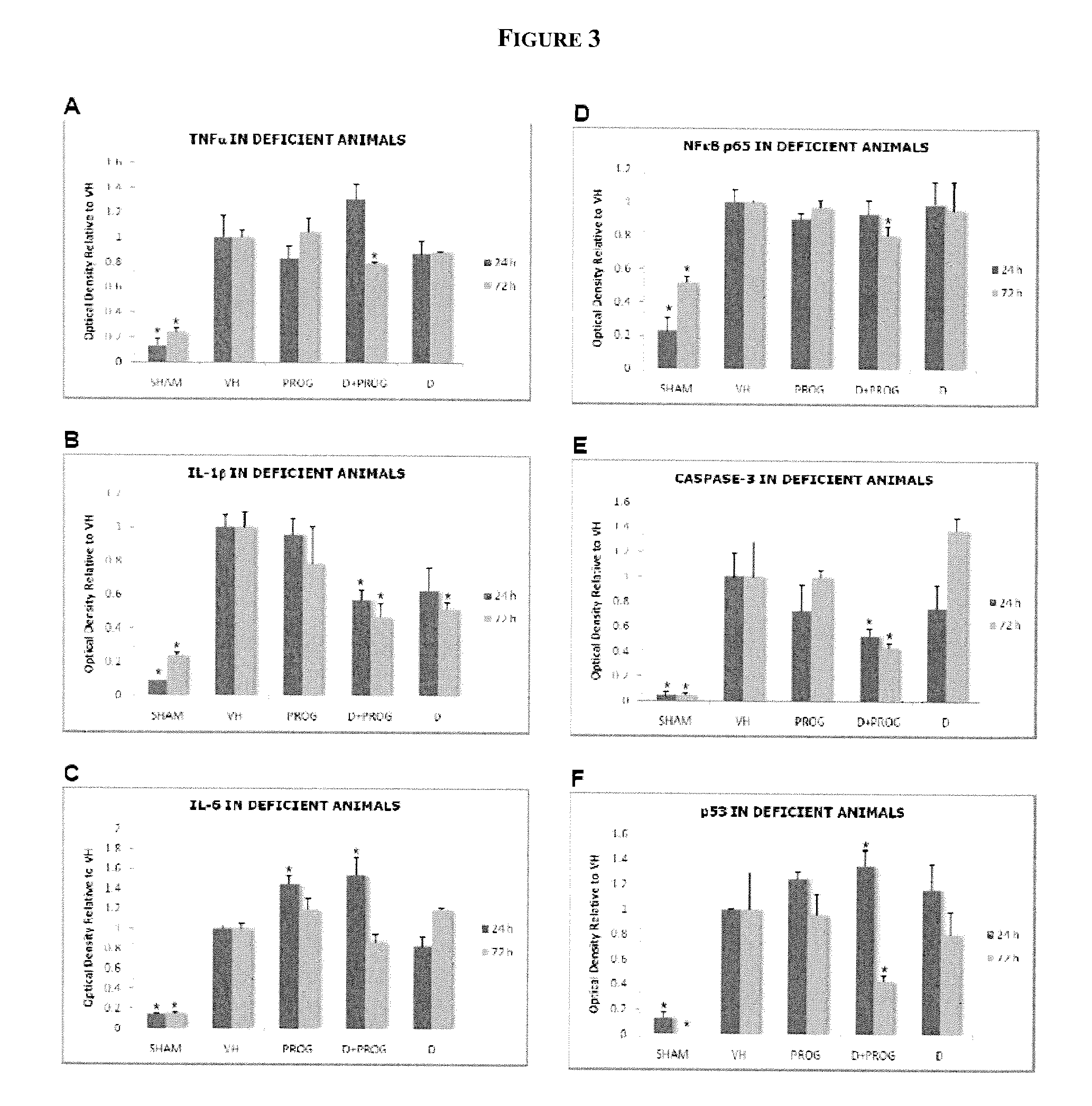
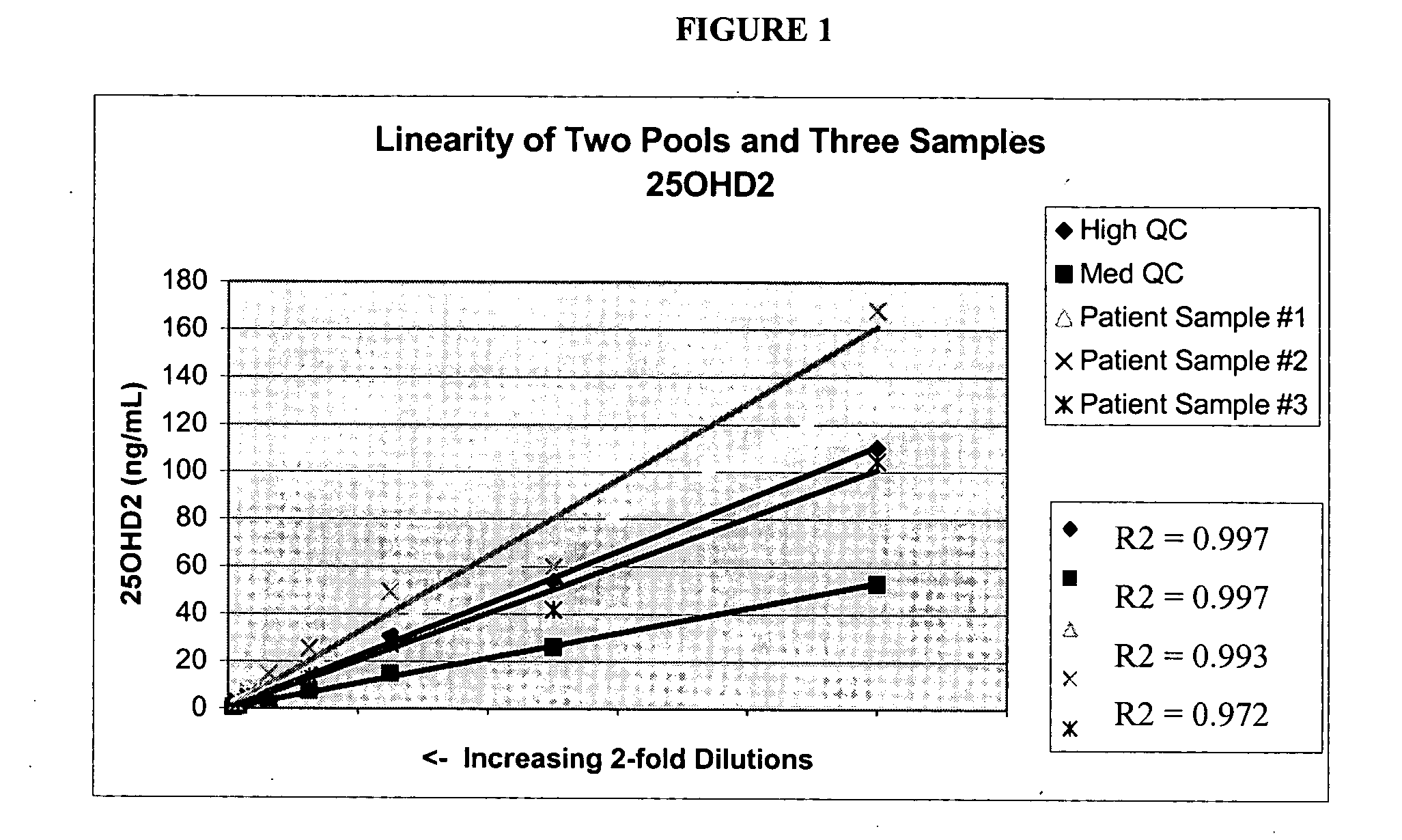
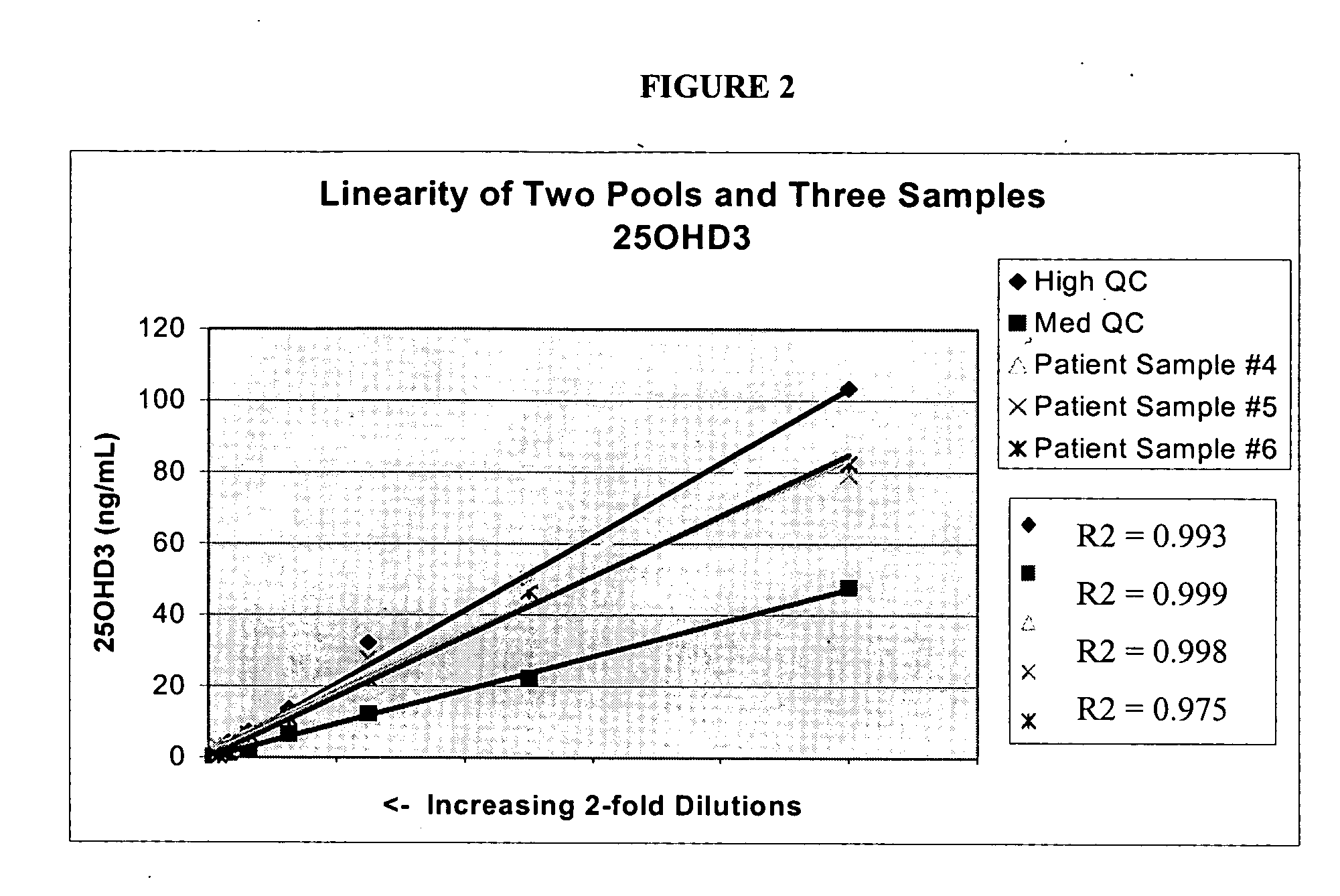
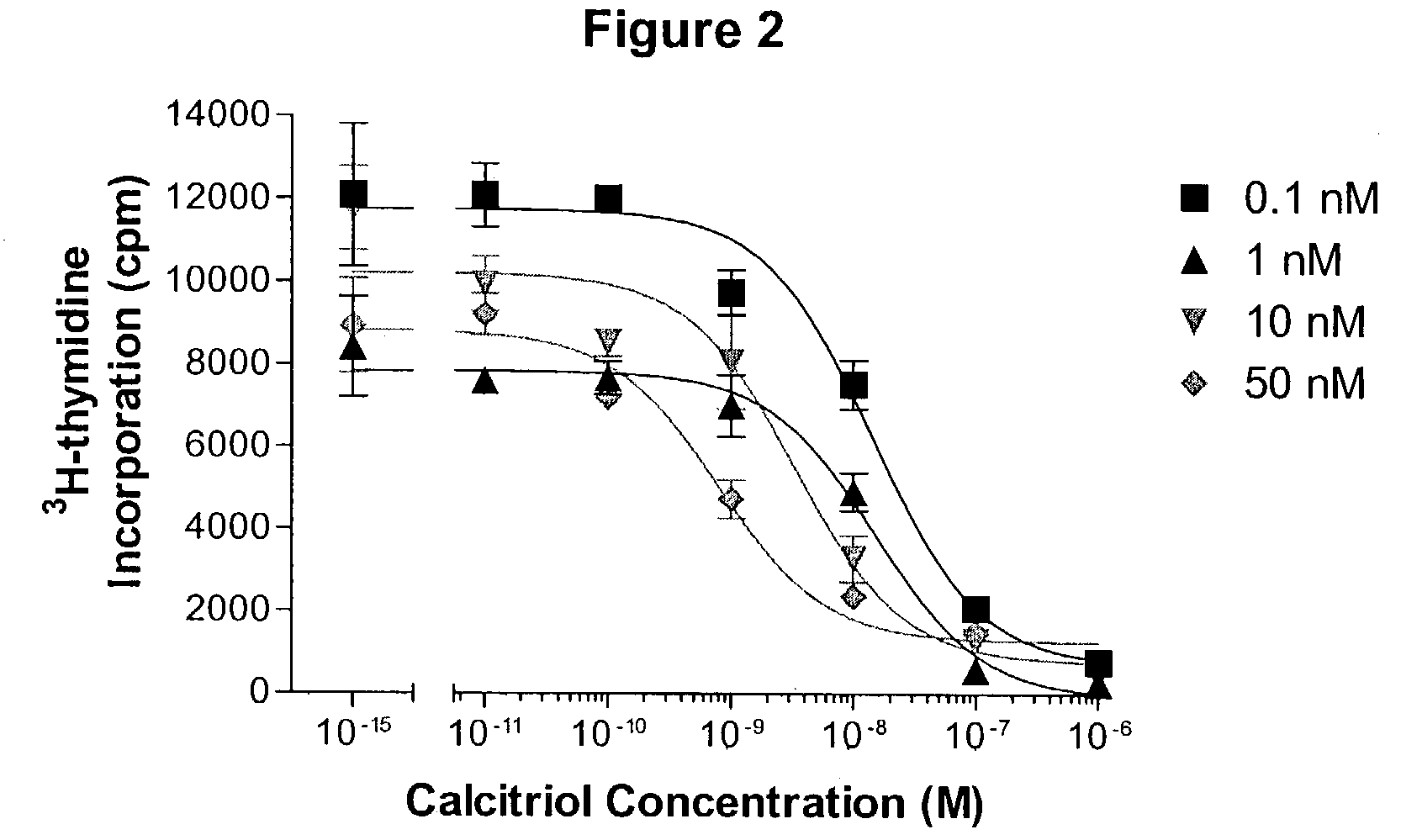
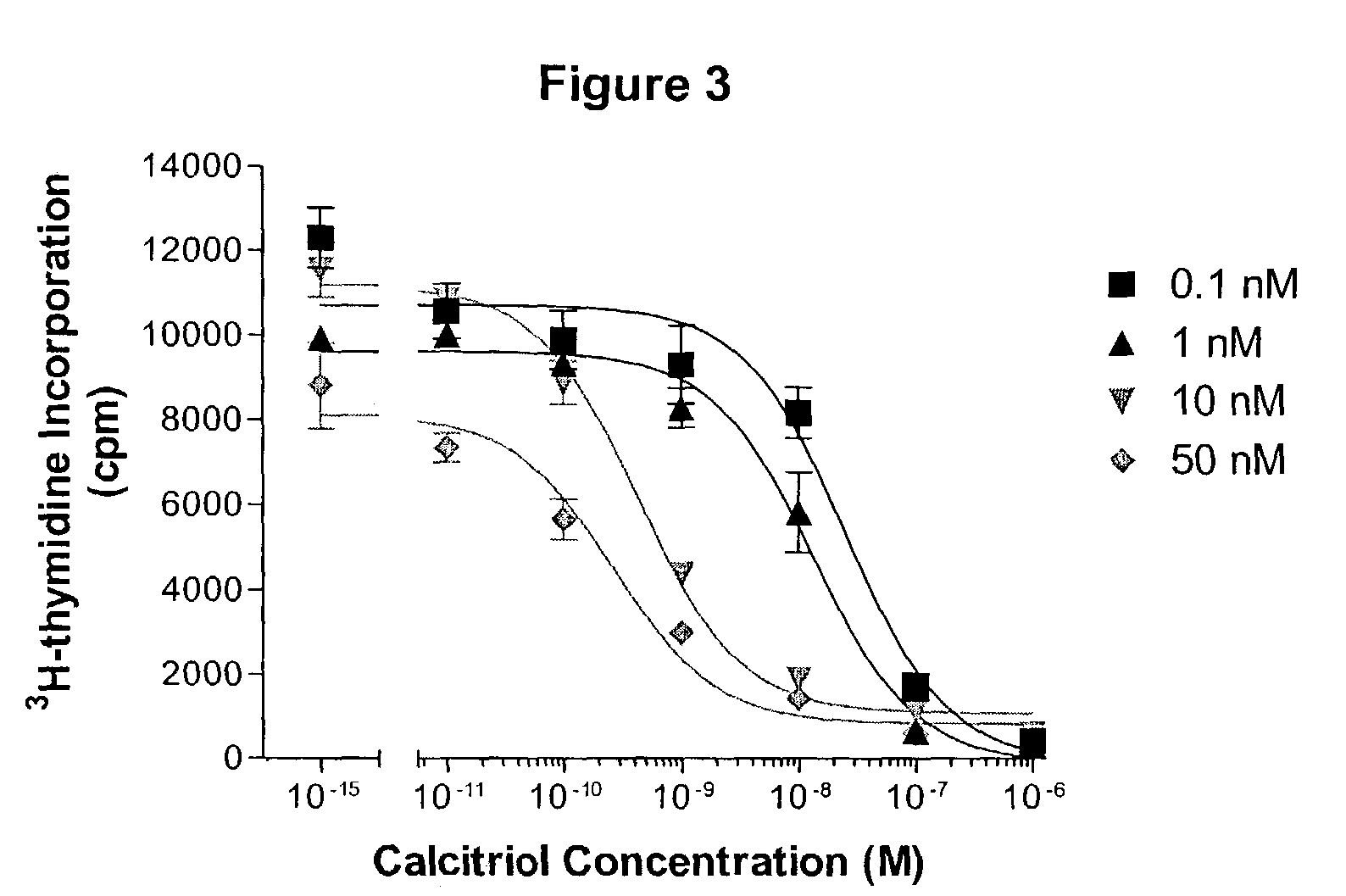
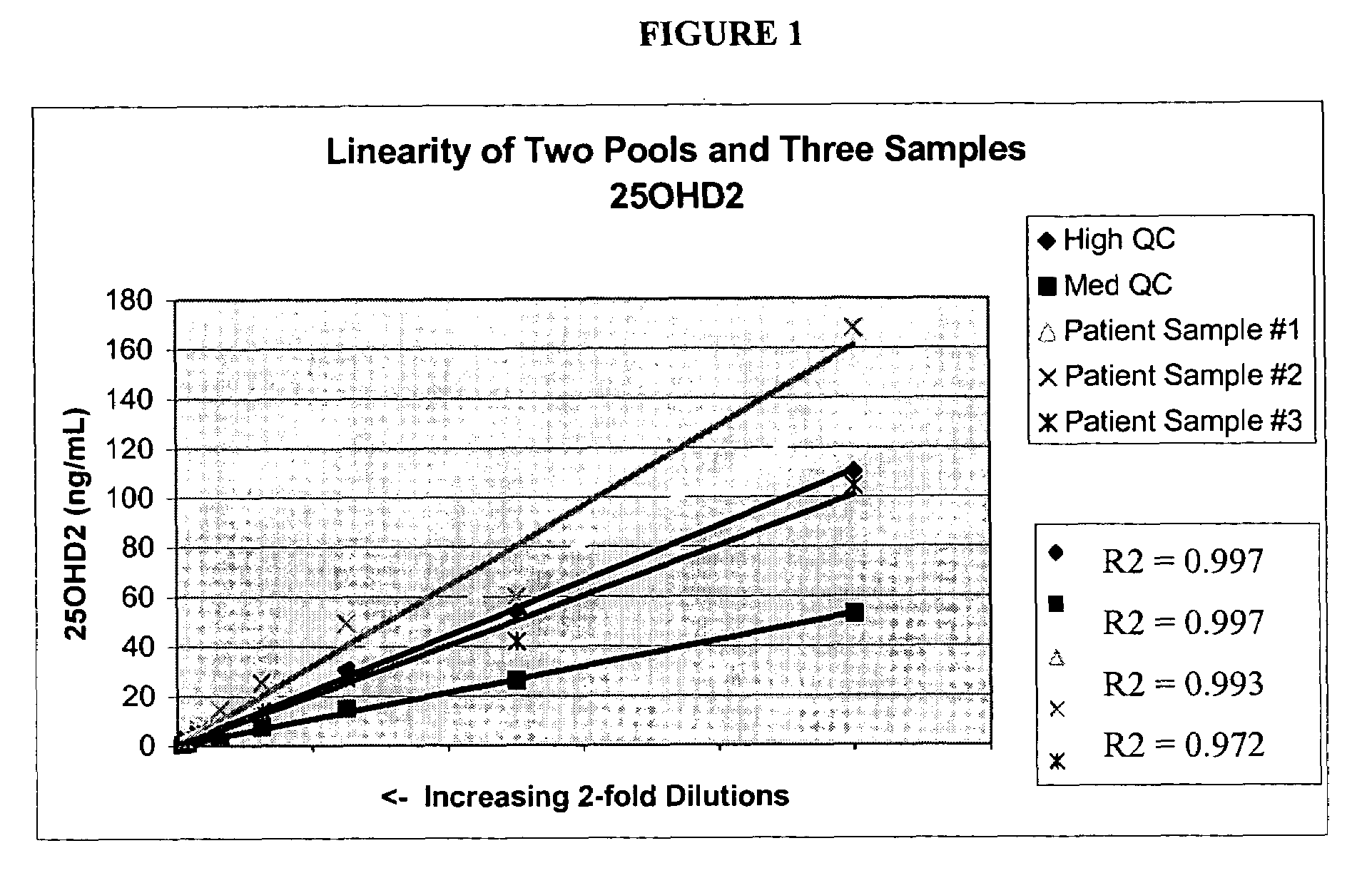
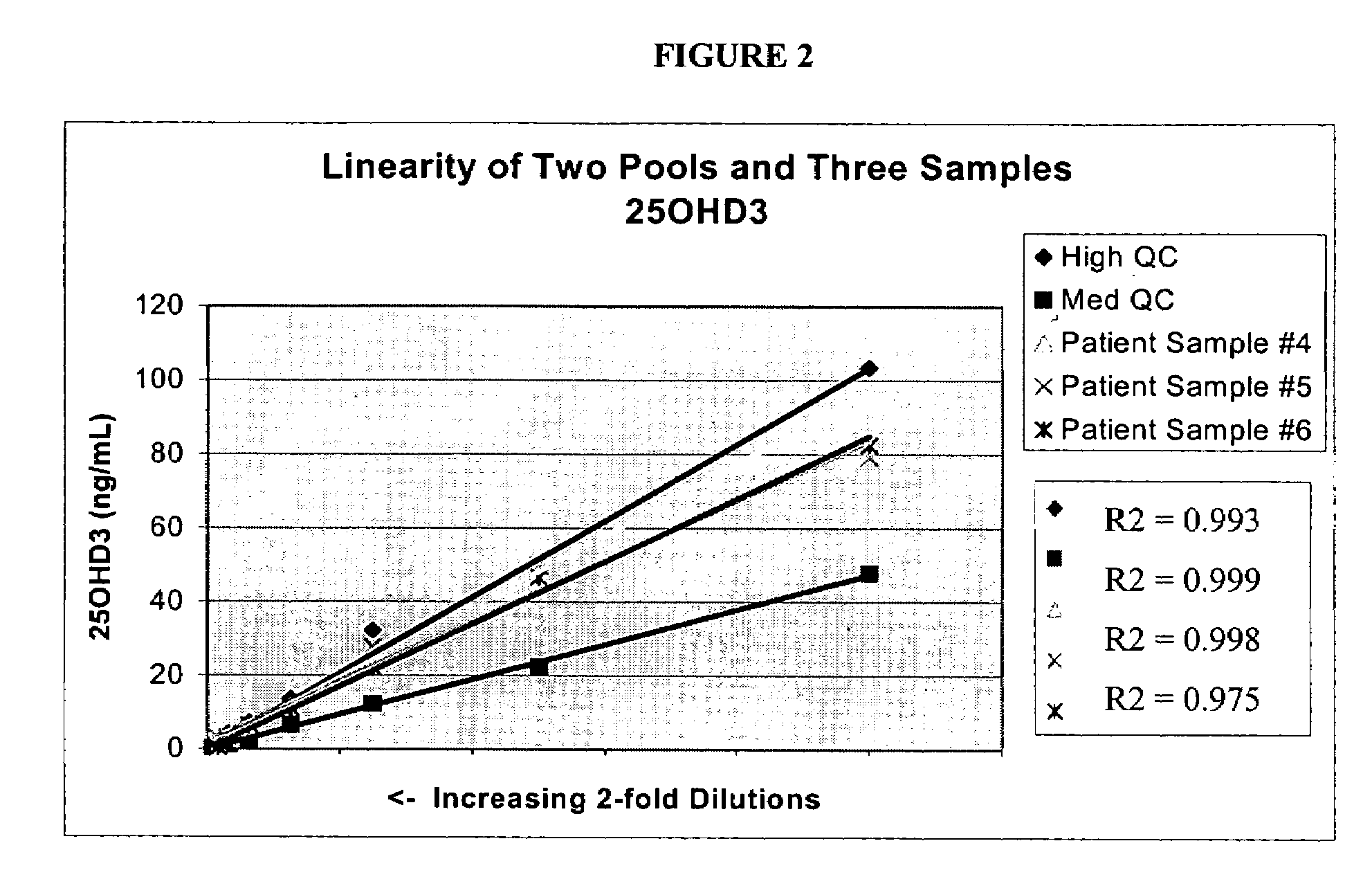
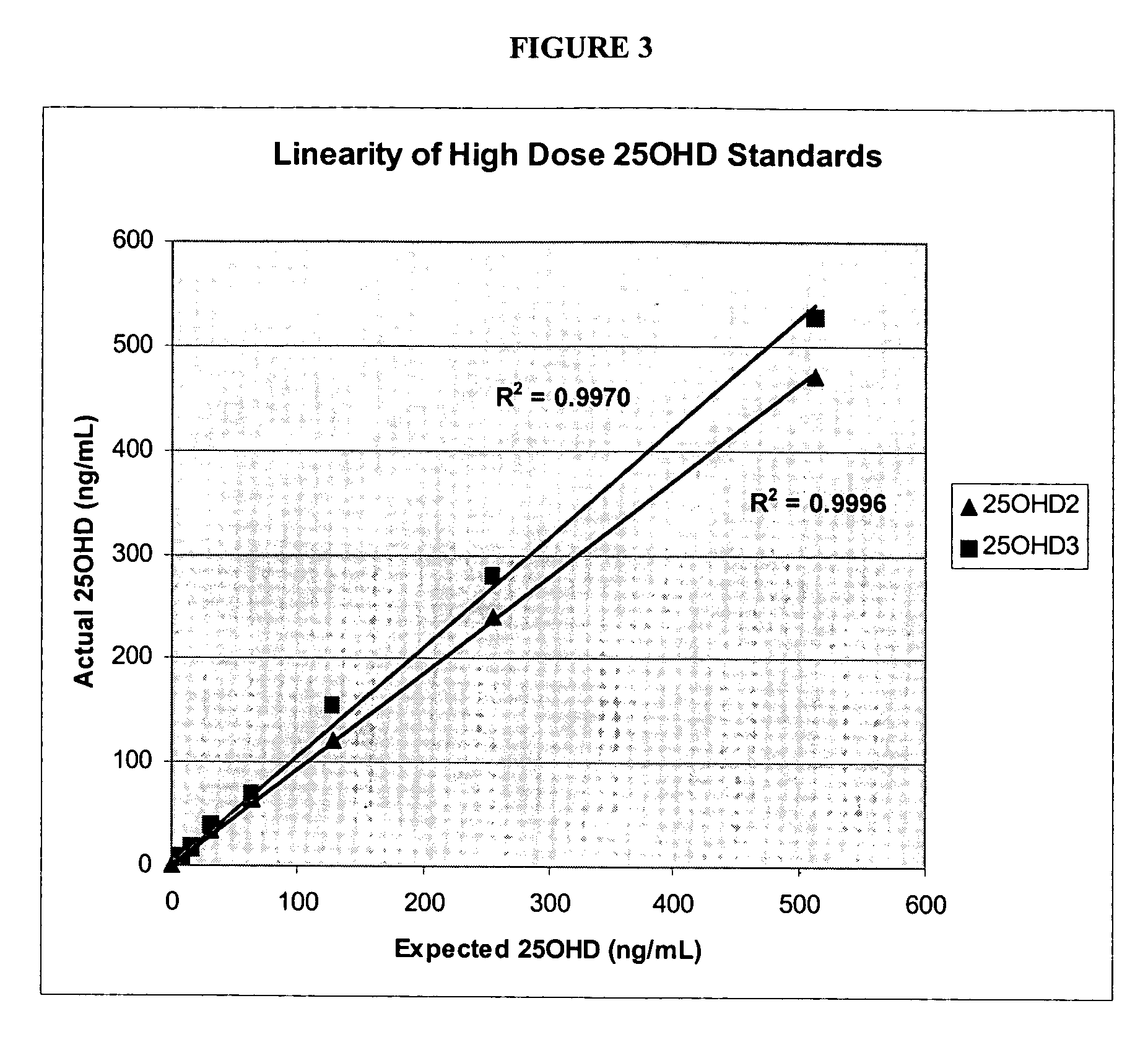
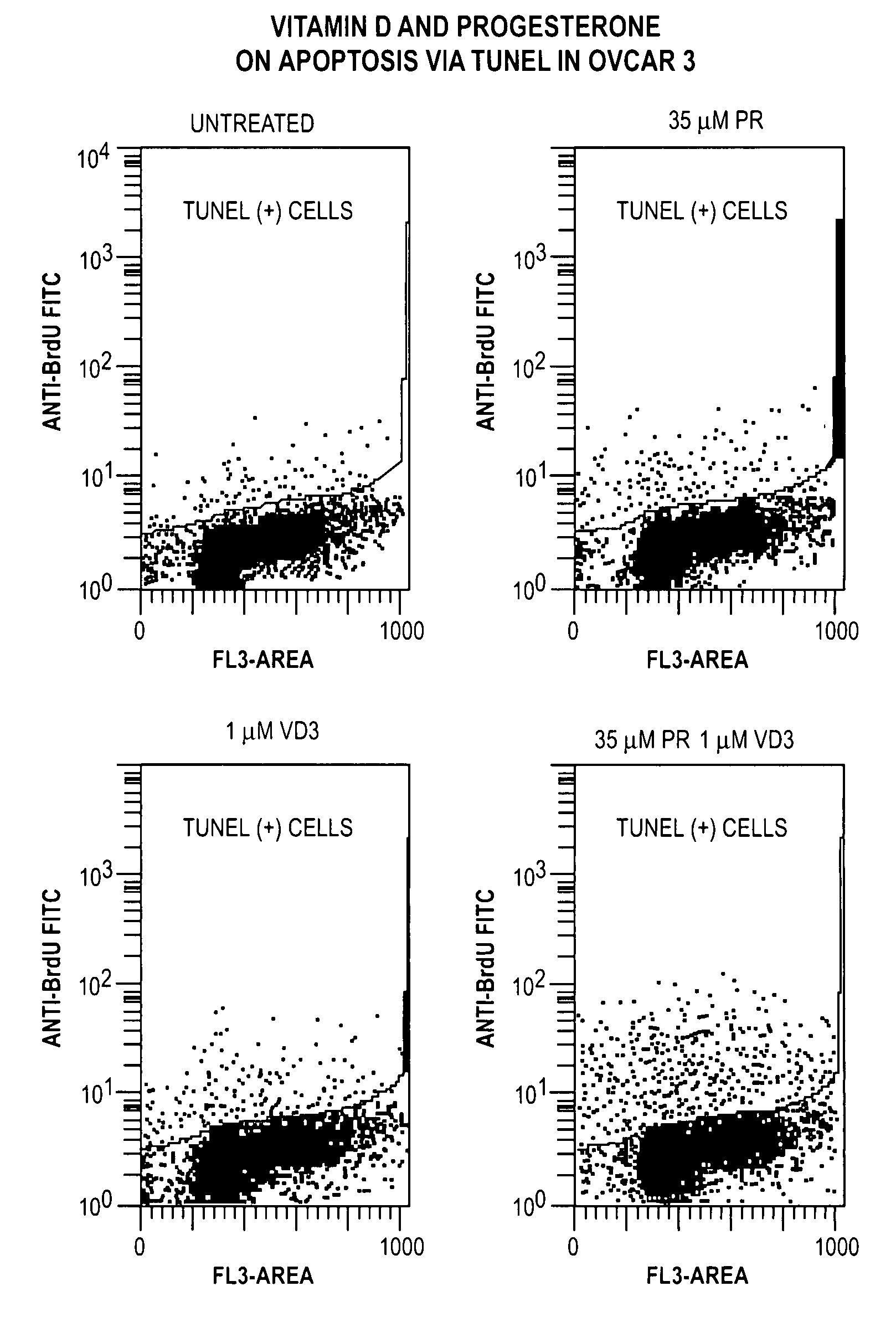
Patents
Literature
1999 results about "Vitamin D+Metabolites" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
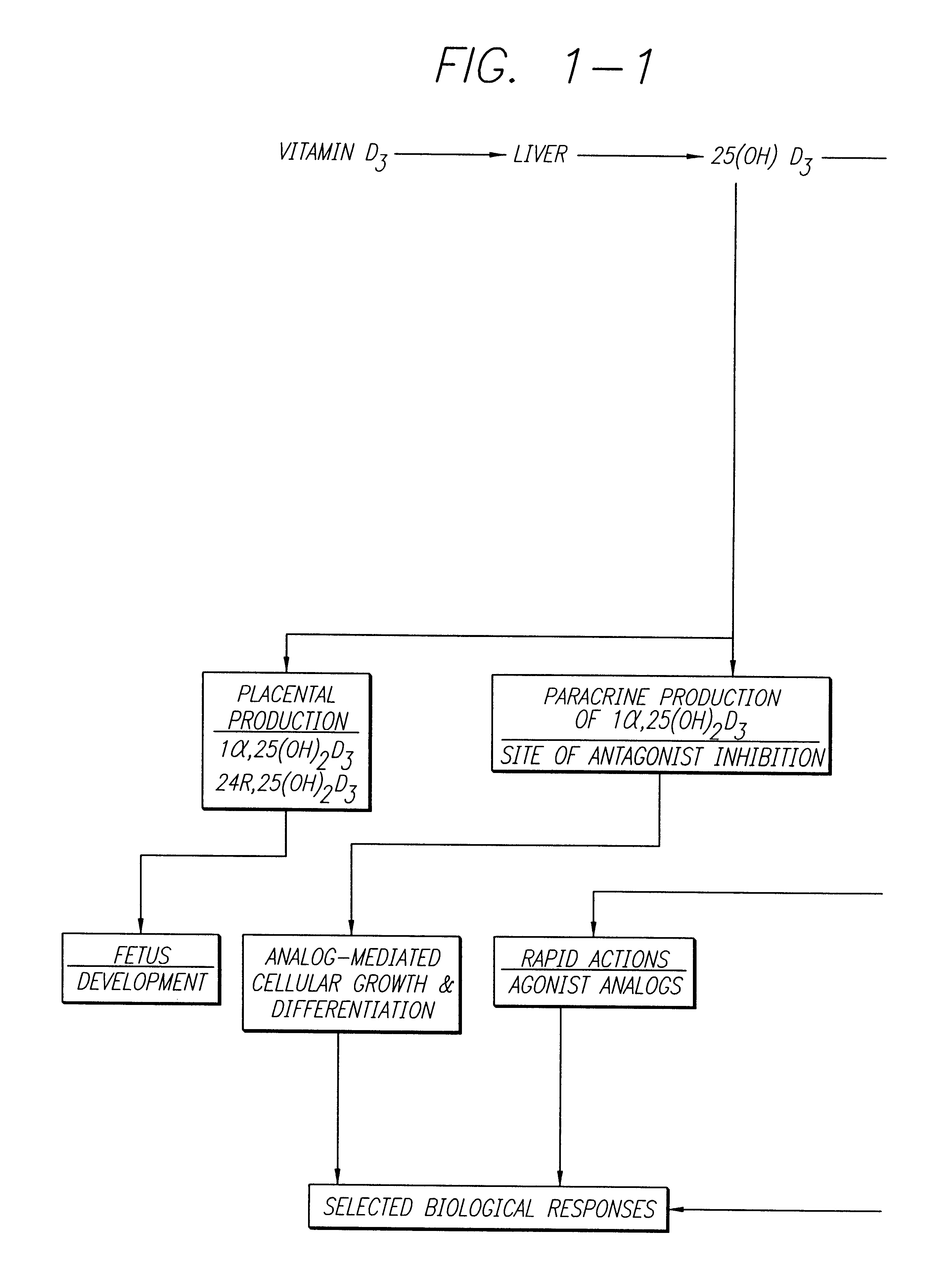
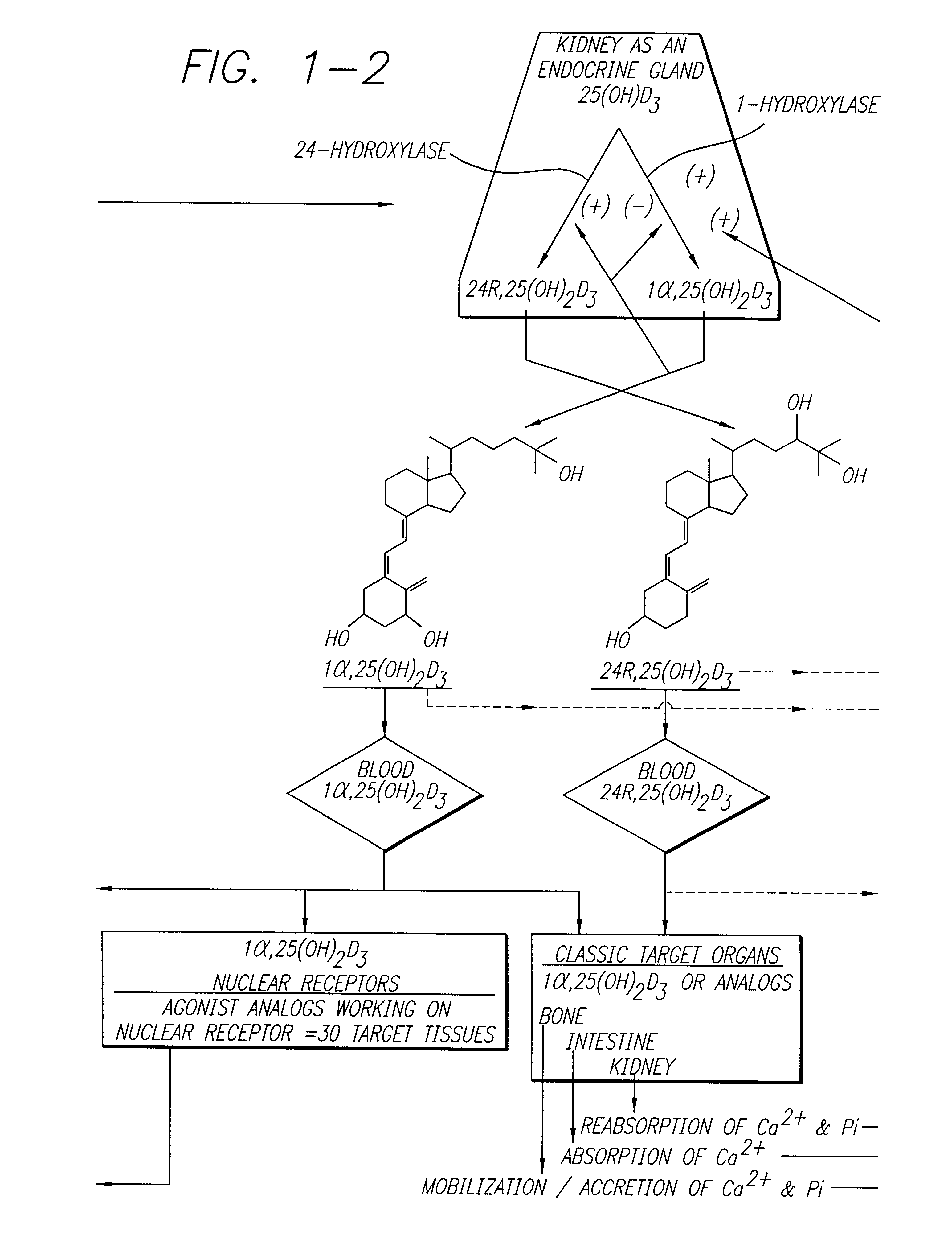
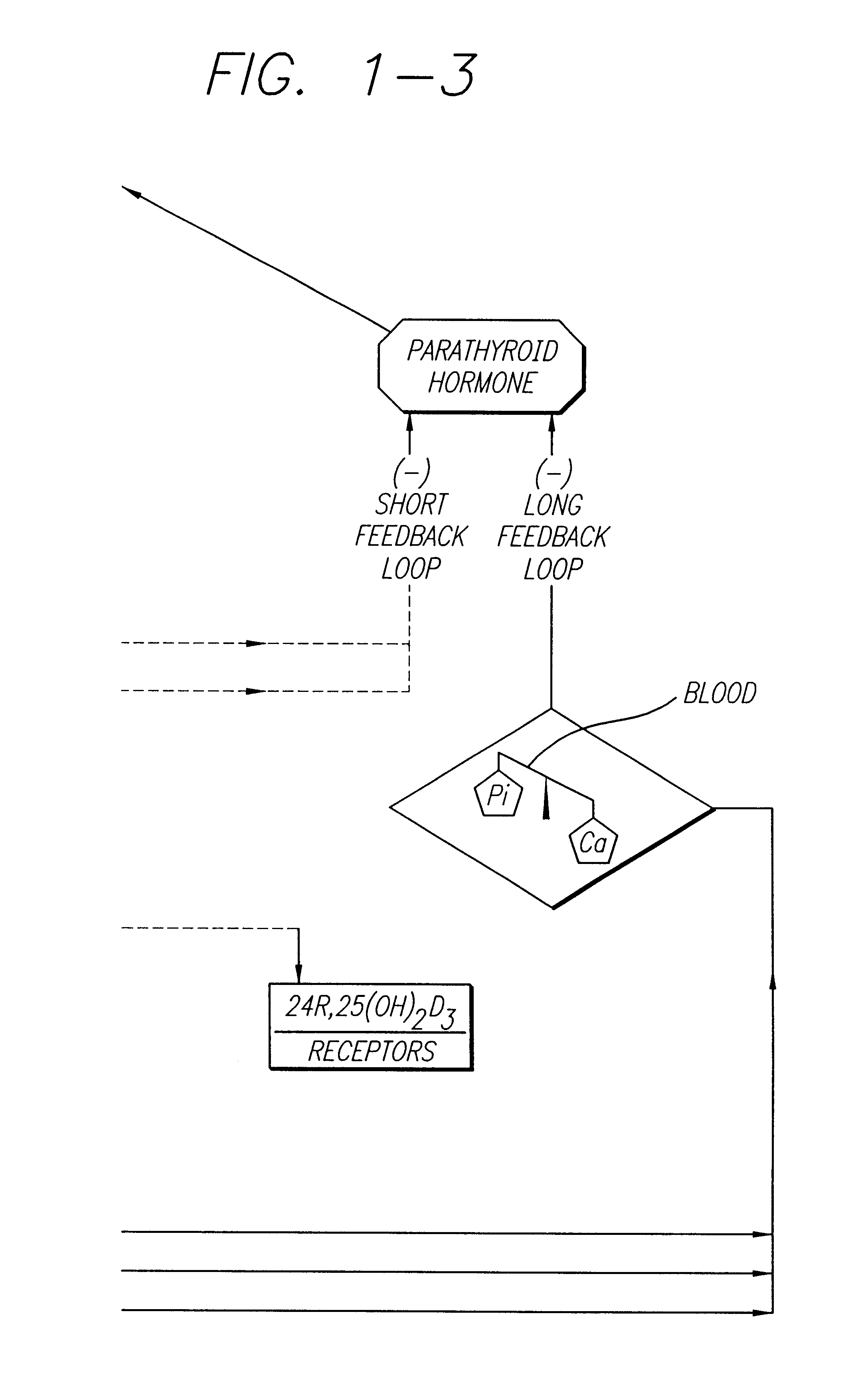
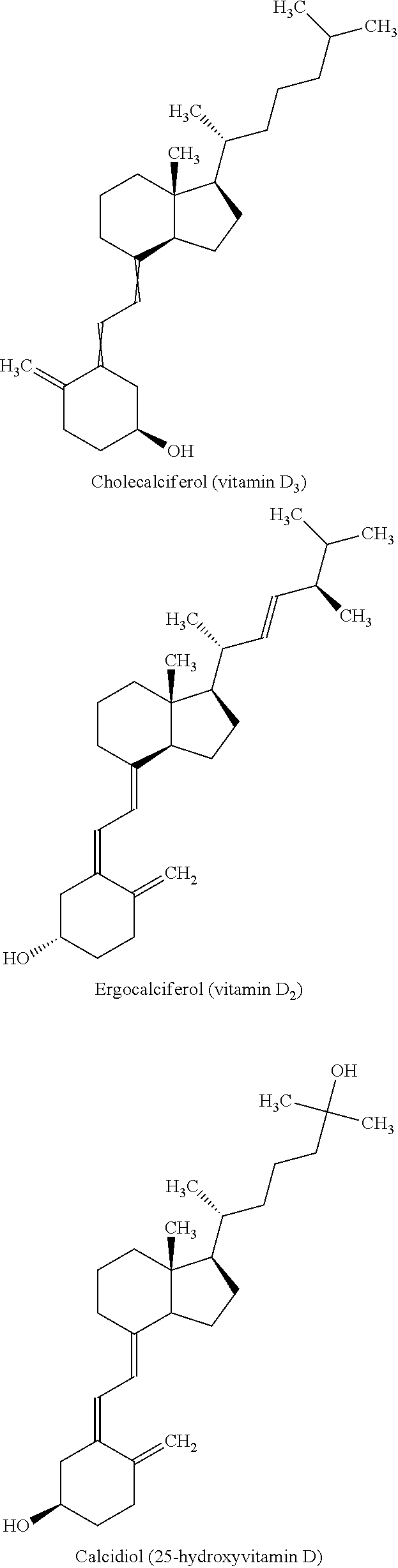
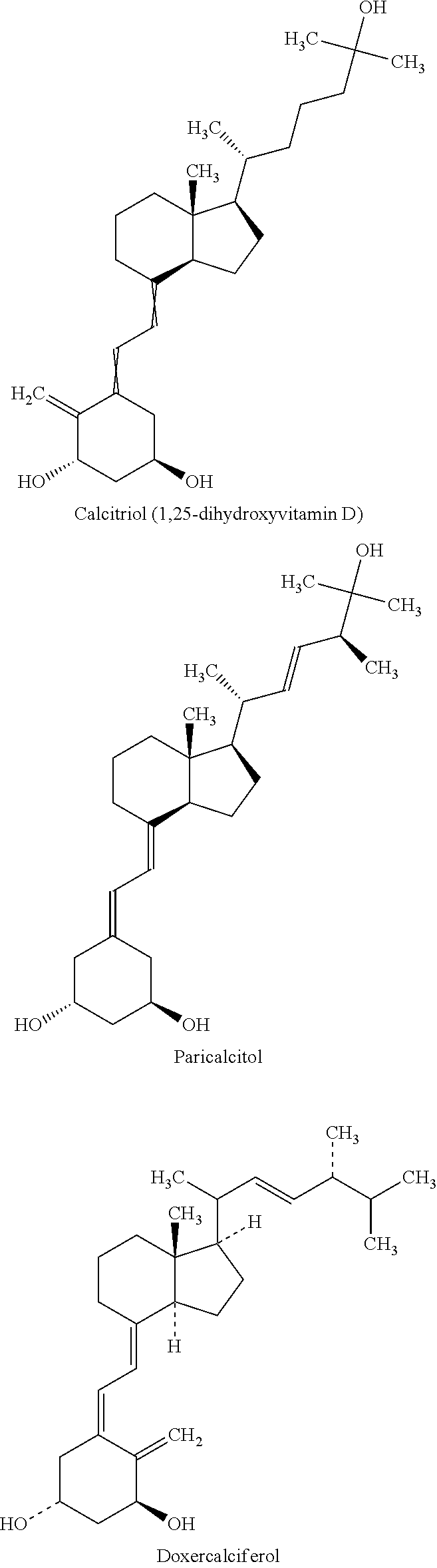
Cholecalciferol is converted in the liver to calcifediol (25-hydroxycholecalciferol); ergocalciferol is converted to 25-hydroxyergocalciferol. These two vitamin D metabolites (called 25-hydroxyvitamin D or 25(OH)D) are measured in serum to determine a person's vitamin D status.
Composition for promoting healthy bone structure
InactiveUS6447809B1Increase bone densityPrevents radial bone lossBiocideHeavy metal active ingredientsVitamin CRegimen
A dietary supplement for benefitting human bone health includes a calcium source, a source of vitamin D activity, and an osteoblast stimulant. A preferred calcium source is microcrystalline hydroxyapatite, which also contains protein (mostly collagen), phosphorus, fat, and other minerals. A preferred source of vitamin D activity is cholecalciferol, and a preferred osteoblast stimulant is ipriflavone. In addition to these basic ingredients, the composition can further include various other minerals known to occur in bone, vitamin C, and glucosamine sulfate, all of which exert beneficial effects on growth and maintenance of healthy bone. A method for benefitting human bone health involves administering a daily regimen of the dietary supplement.
Owner:PHOENIX DICHTUNGSTECHN +1
Vitamin D3 mimics
The present invention relates to non-secosteroidal compounds which activate and modulate the vitamin D receptor (VDR). Because compounds of the present invention display many of the beneficial properties of 1,25(OH)2D3, but with reduced calcium mobilization effects, they may be used advantageously to treat and prevent conditions that show vitamin D sensitivity. Such disease states typically show abnormal calcium regulatory, abnormal immune responsive, hyperproliferative, and / or neurodegenerative characteristics.
Owner:LIGAND PHARMA INC
Sprayable compositions comprising a combination of pharmaceutical actives and an oily phase
InactiveUS20050281749A1Good acceptability and toleranceChemically stableBiocideAerosol deliveryDermatological disordersOil phase
Sprayable, anhydrous and physically / chemically stable dermatological / pharmaceutical compositions, well suited for the treatment of a variety of dermatological disorders, notably psoriasis, contain: a) a therapeutically effective amount of a solubilized corticoid, notably dissolved clobetasol propionate; b) a therapeutically effective amount of a solubilized vitamin D derivative, notably dissolved calcitriol; and c) an oily phase which comprises one or more oils; formulated into d), a sprayable and topically applicable, dermatologically / pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
Methods of neuroprotection using neuroprotective steroids and a vitamin d
InactiveUS20110306579A1Inhibiting neurodegenerationPromote physical recoveryBiocideOrganic active ingredientsNervous systemProgesterones
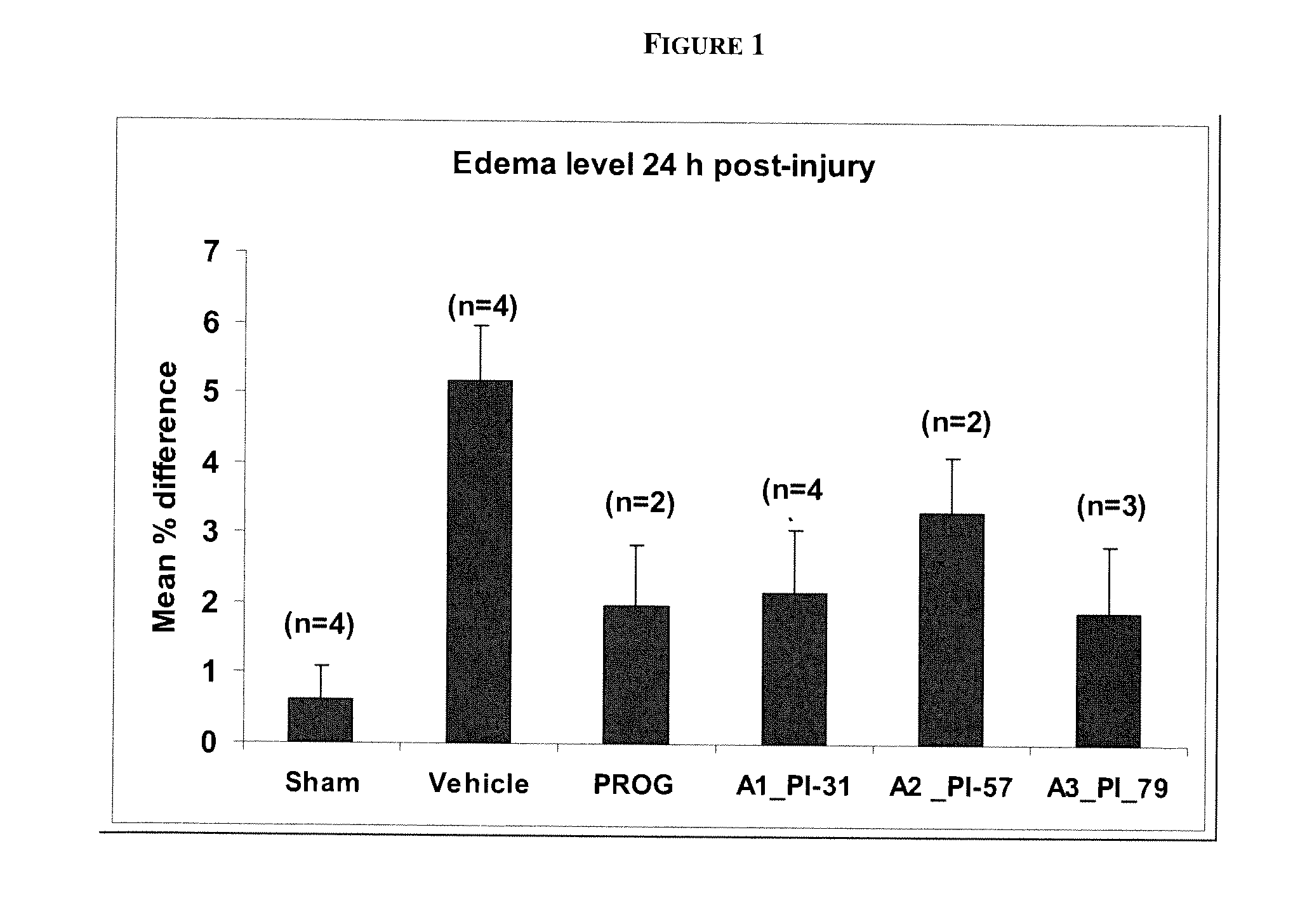
Described herein are compositions and methods for treating or preventing nervous system injury. In particular, the methods and compositions relate to the use of at least one neuroprotective steroid, such as progesterone, and vitamin D.
Owner:EMORY UNIVERSITY
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Methods for detecting vitamin D metabolites
ActiveUS20060228808A1Increased turbulenceEasy to separateComponent separationMass spectrometric analysisMetaboliteMass Spectrometry-Mass Spectrometry
Provided are methods of detecting the presence or amount of a vitamin D metabolite in a sample using mass spectrometry. The methods generally comprise ionizing a vitamin D metabolite in a sample and detecting the amount of the ion to determine the presence or amount of the vitamin D metabolite in the sample. Also provided are methods to detect the presence or amount of two or more vitamin D metabolites in a single assay.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Functional vitamin D derivatives and a method for determining 25-hydroxy-vitamin D and 1alpha, dihydroxy-vitamin D
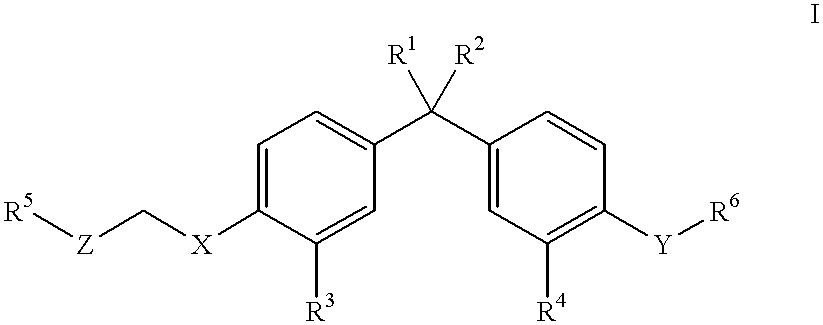
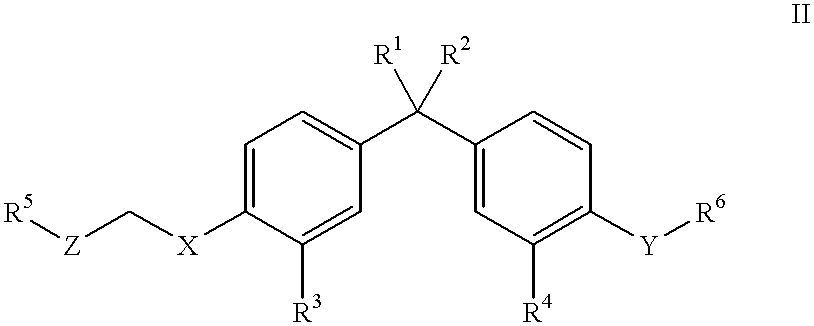
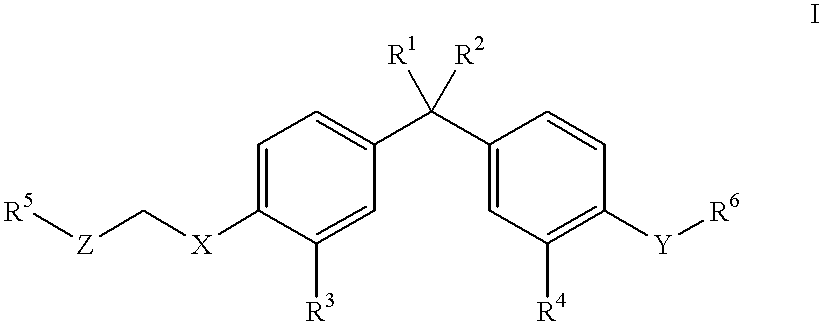
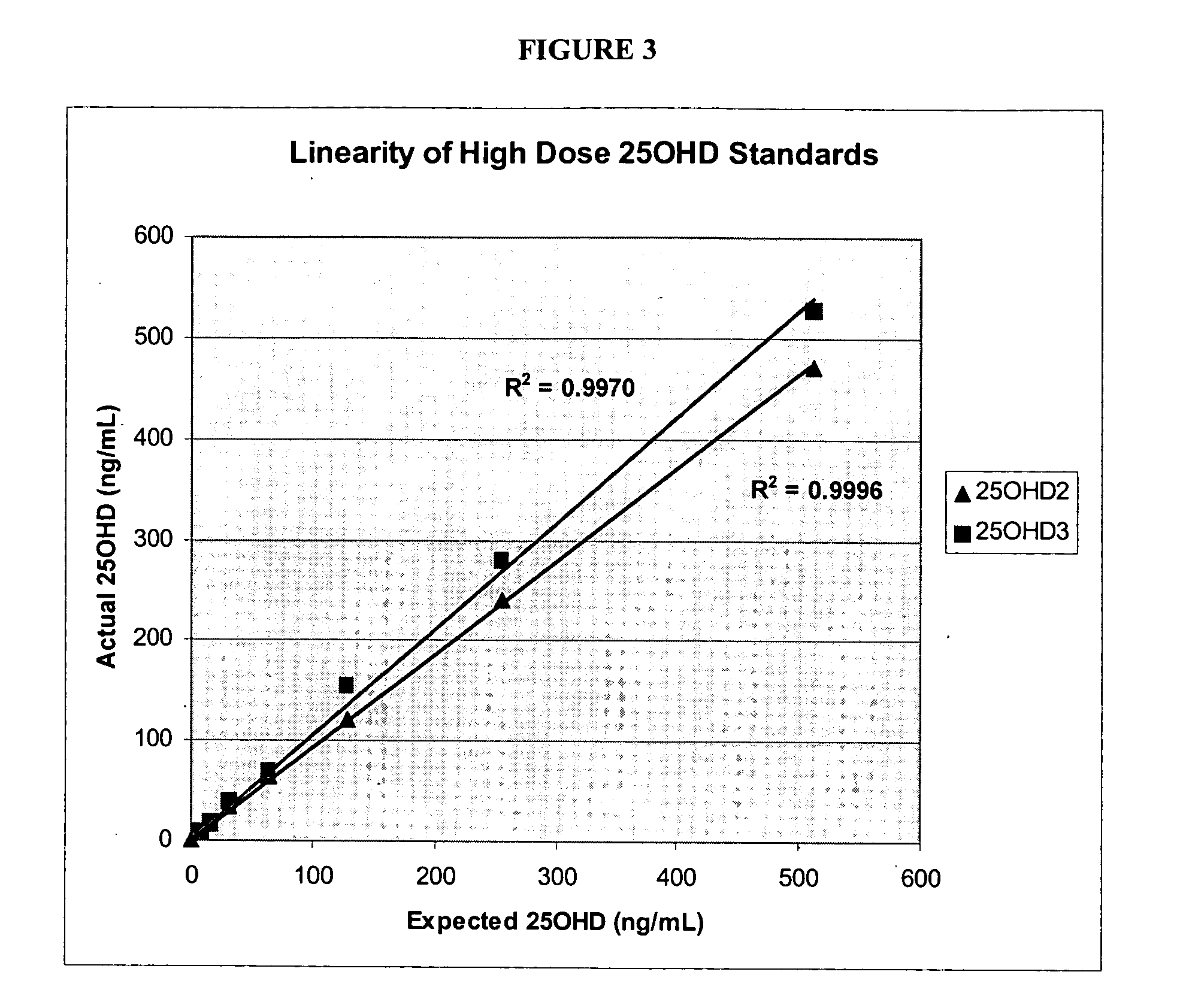
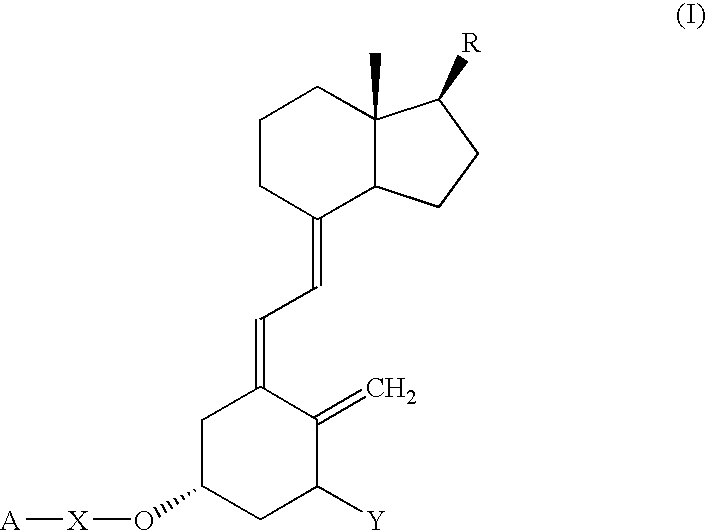
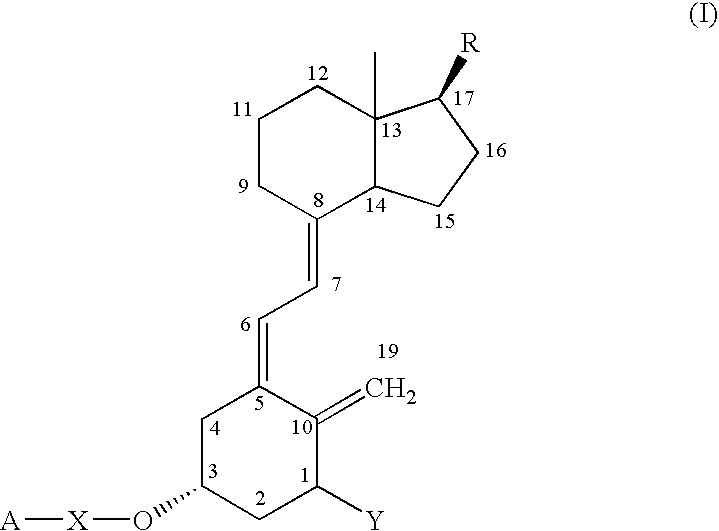
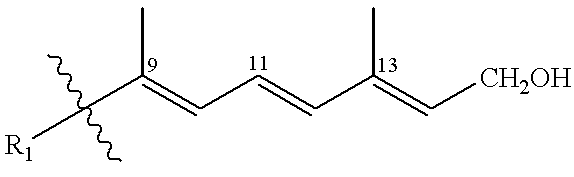
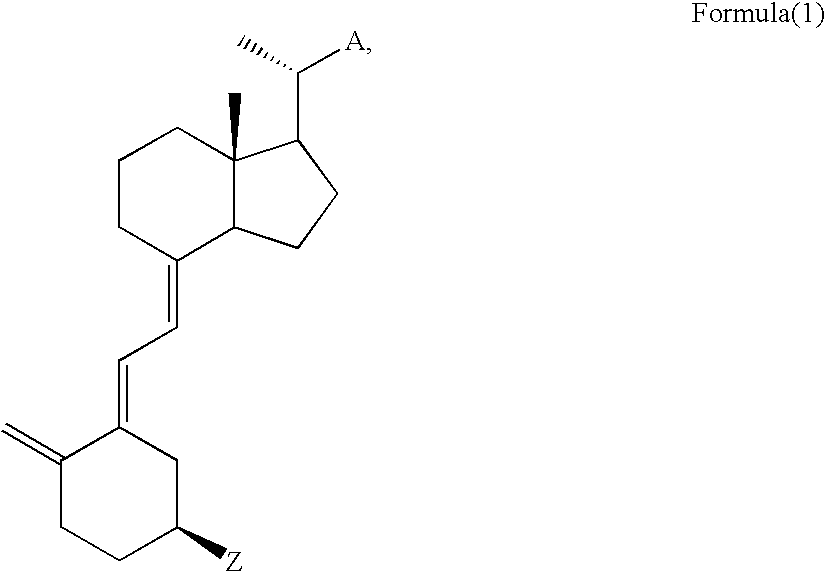
Vitamin D compounds of formula (I) with a label attached to a spacer group in the 3 position are disclosed.In the above formula (I), X is an optionally substituted hydrocarbon group with a length of 0.8-4.2 nm, optionally containing the heteroatoms S, O, N or P; Y is H or OH; A is a label capable of binding with high affinity to a protein; R is an optionally substituted hydrocarbon side chain of a D vitamin or a D vitamin metabolite. Also disclosed is the preparation of formula (I).
Owner:IMMUNDIAGNOSTIK +1
Vitamin D analogs for obesity prevention and treatment
InactiveUS20050119242A1Reducing body fatIncrease transcriptionBiocideOrganic active ingredientsObesity preventionVitamin D Analog
Methods for treating and preventing obesity, inhibiting adipocyte differentiation, inhibiting increased SCD-1 gene transcription, and / or reducing body fat in a subject include administering at least one analog of 1α,25-dihydroxyvitamin D3 or 1α,25-dihydroxyvitamin D2 or a pharmaceutical composition that includes such an analog to a subject in need thereof. The analog may be a 19-nor vitamin D analog such as a compound of formula IA, a compound of formula IB, or a mixture thereof where the variables R1, R2, and R3 have the values described herein.
Owner:WISCONSIN ALUMNI RES FOUND
Methods useful for remodeling maxillofacial bone using light therapy and a functional appliance
Methods are provided for regulating bone remodeling or tooth movement, comprising allowing a functional appliance to exert a force on oral or maxillofacial bone, muscle, or soft tissue, or one or more teeth of a patient in need thereof; and administering an effective amount of light to the oral or maxillofacial bone, muscle, or soft tissue, or one or more teeth, wherein the light is administered before, during, or after the force is exerted. Methods are also provided for regulating bone remodeling, comprising administering an effective amount of vitamin D to an oral or maxillofacial bone, muscle, or soft tissue, or to one or more teeth of a patient in need thereof; and administering an effective amount of light to the oral or maxillofacial bone, muscle, or soft tissue, or to the one or more teeth. Apparatuses useful for providing light therapy and / or vitamin D is also provided.
Owner:BIOLUX RES HLDG INC +1
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
InactiveUS20050101576A1Minimizing and avoiding effectUseful in treatmentOrganic active ingredientsBiocideActive agentHigh doses
Methods of treating MDS, or ameliorating a symptom thereof, are disclosed. Specific methods encompass the administration of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Other methods include intermittent administration of a high dose of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Such intermittent administration allows high doses of the vitamin D compounds to be administered while minimizing or eliminating hypercalcemia.
Owner:NOVACEA INC
Methods for detecting dihydroxyvitamin d metabolites by mass spectrometry
ActiveUS20090137056A1Material analysis by electric/magnetic meansBiological testingMetaboliteMass Spectrometry-Mass Spectrometry
Provided are methods of detecting the presence or amount of a dihydroxyvitamin in D metabolite in a sample using mass spectrometry. The methods generally comprise ionizing a dihydroxyvitamin D metabolite in a sample and detecting the amount of the ion to determine the presence or amount of the vitamin D metabolite in the sample. In certain preferred embodiments the methods include immunopurifying the dihydroxyvitamin D metabolites prior to mass spectrometry. Also provided are methods to detect the presence or amount of two or more dihydroxyvitamin D metabolites in a single assay.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Compositions and methods for treating hyperproliferative epidermal diseases
InactiveUS20090131488A1Relieve symptomsHighly efficaciousBiocideHydroxy compound active ingredientsDiseaseVitamin D metabolism
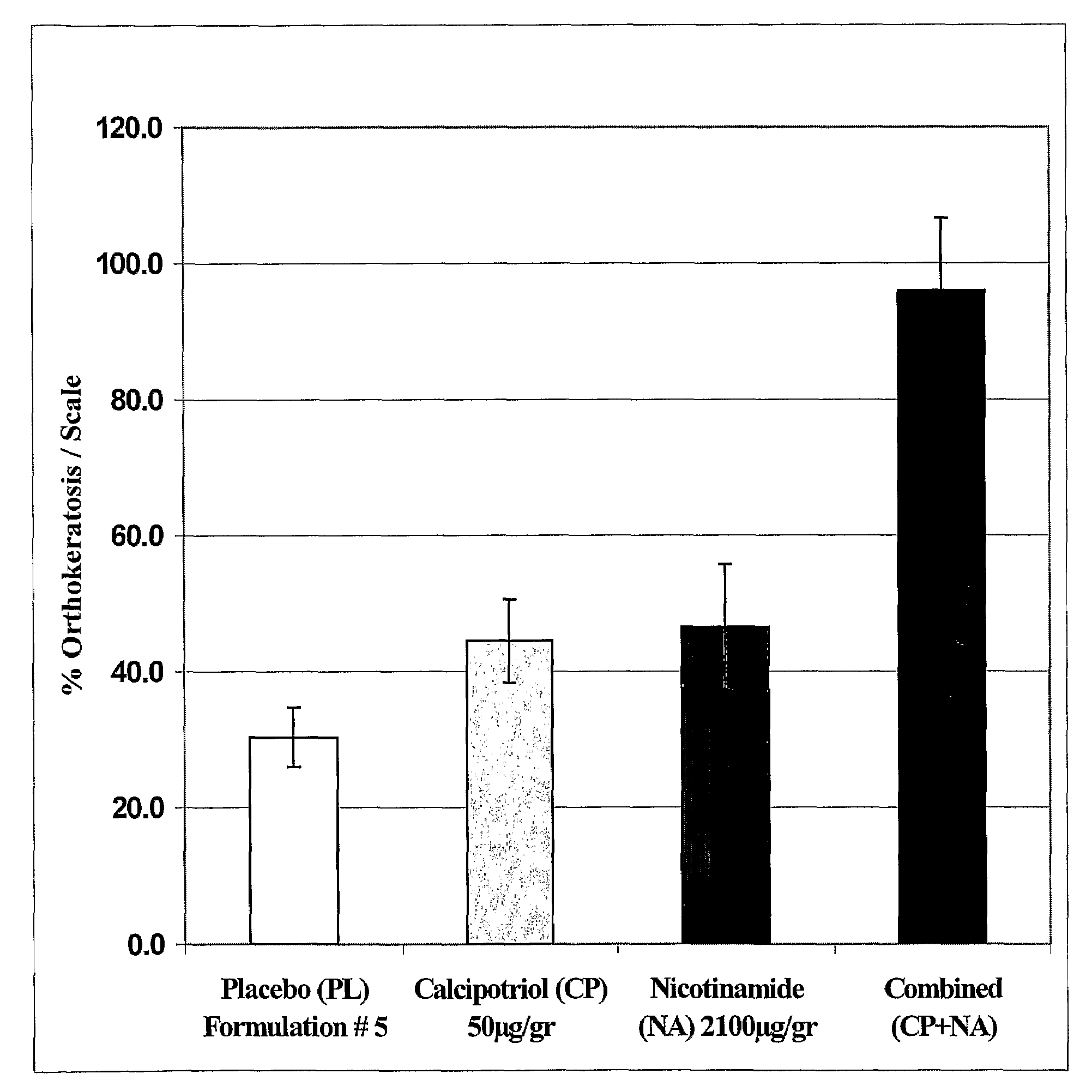
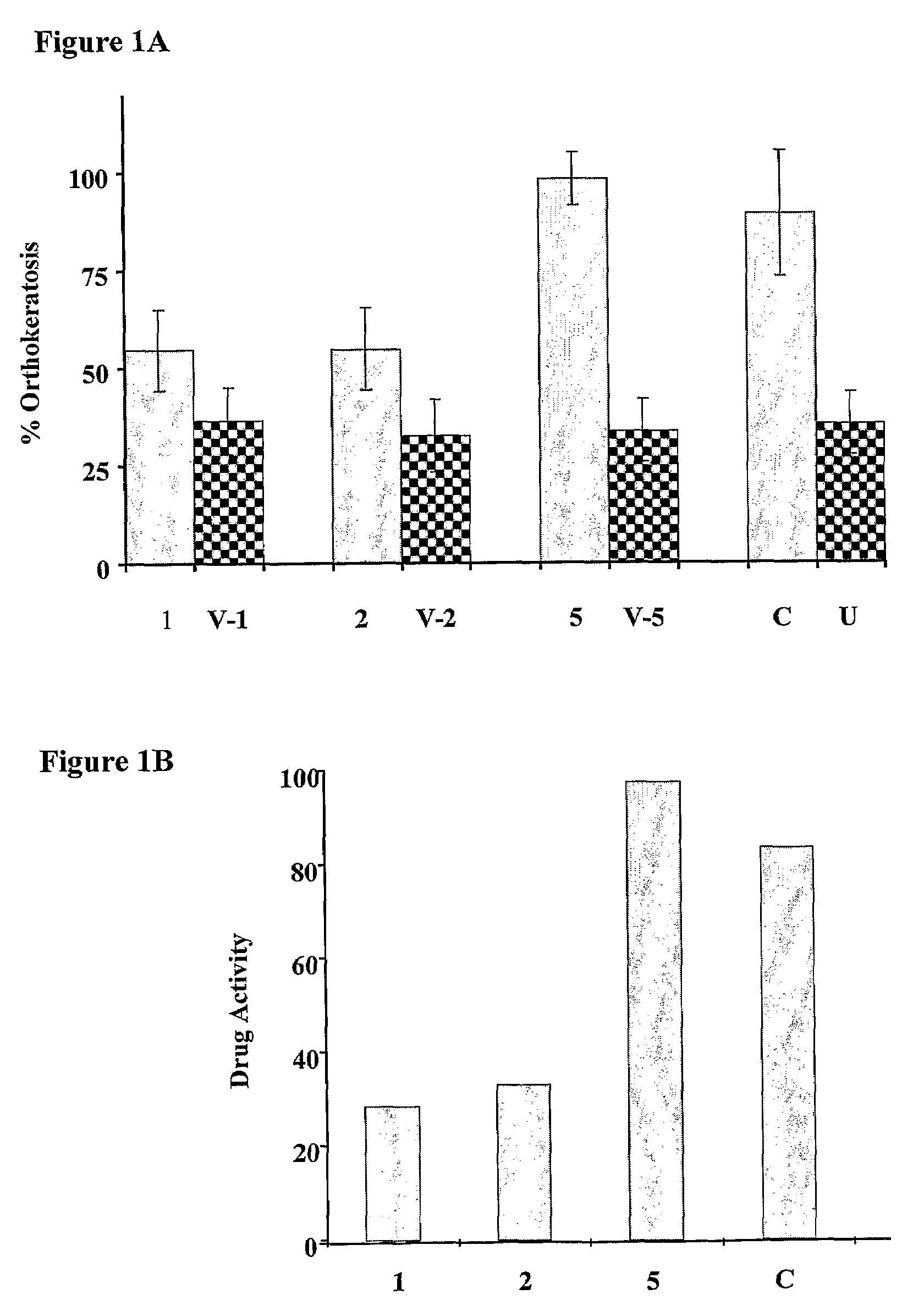
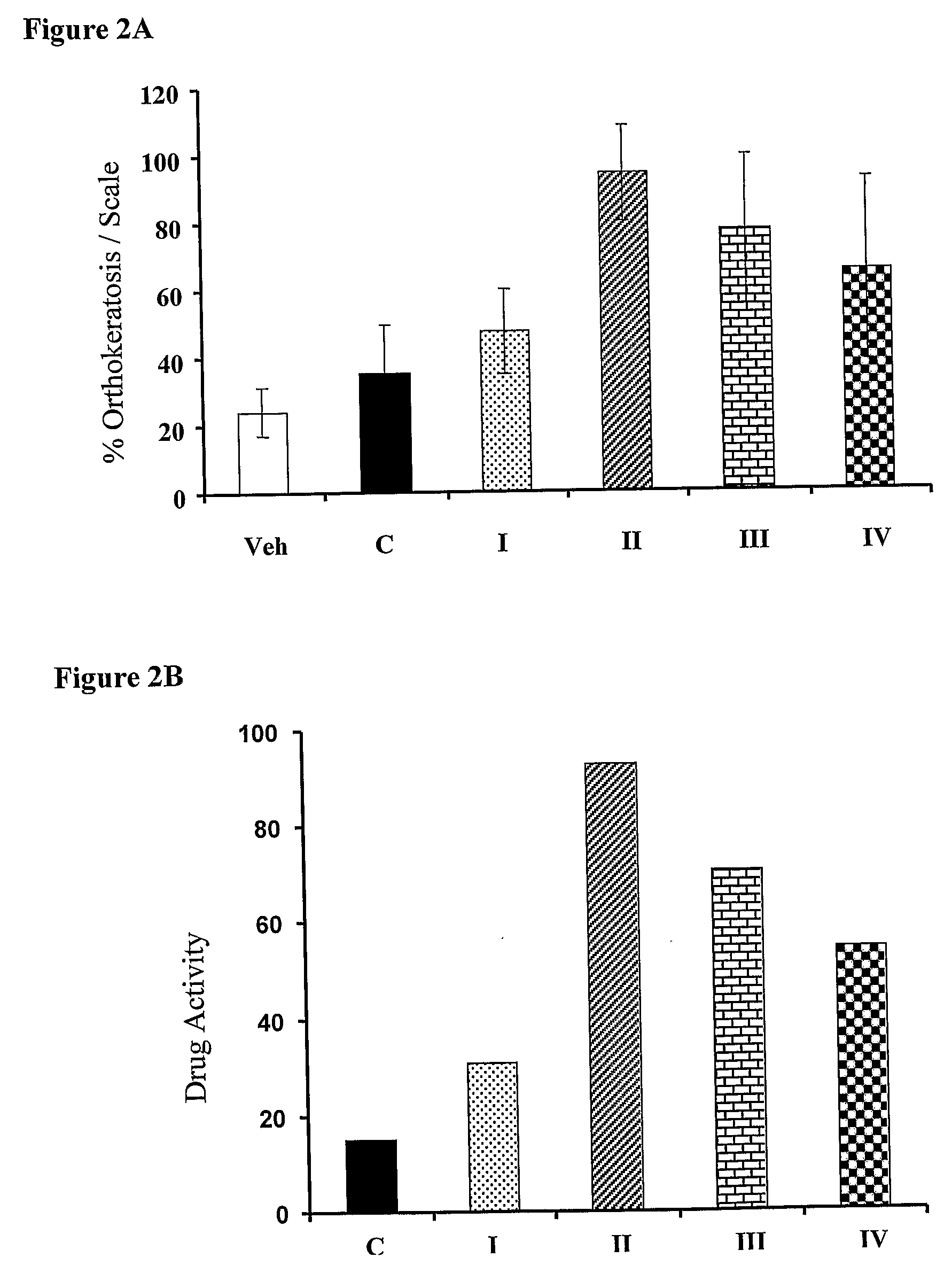
The present invention provides compositions and methods for use in the treatment of hyperproliferative dermal diseases. Specifically, the present invention teaches pharmaceutical compositions for topical administration where the compositions contain nicotinamide and a vitamin D metabolite, calcipotriol, which are particularly effective in treating and in the maintenance treatment of psoriasis and other related dermal disorders and diseases.
Owner:DERMIPSOR LTD
Topical composition
ActiveUS20080234239A1Improve solubilityImprove permeabilityOrganic active ingredientsBiocideChemical compositionDermatology
A composition suitable for topical application comprising a continuous phase and at least one discontinuous phase, said composition comprising at least one polyaphron dispersion, at least one vitamin D or vitamin D analogue and at least one corticosteroid.
Owner:MC2 THERAPEUTICS LTD
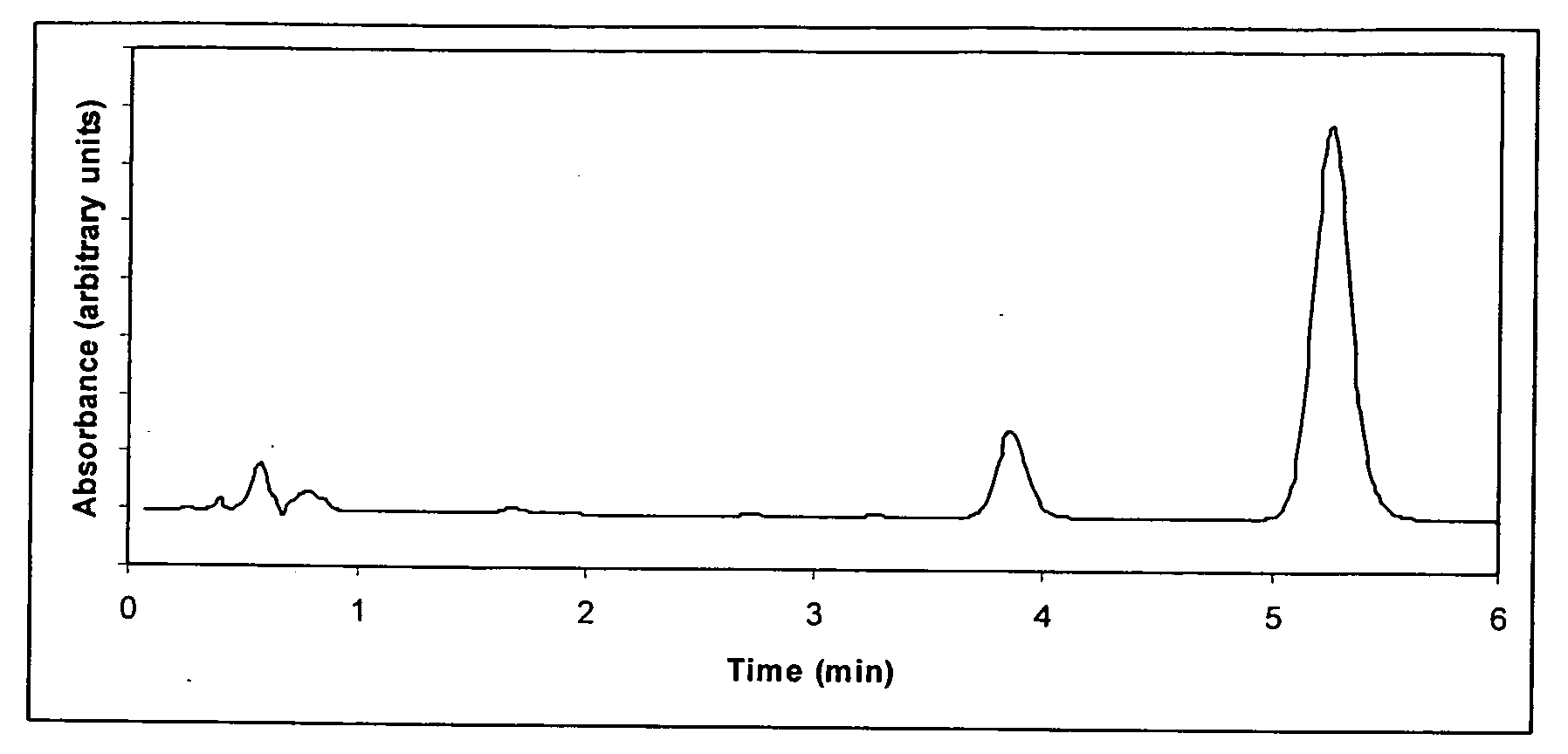
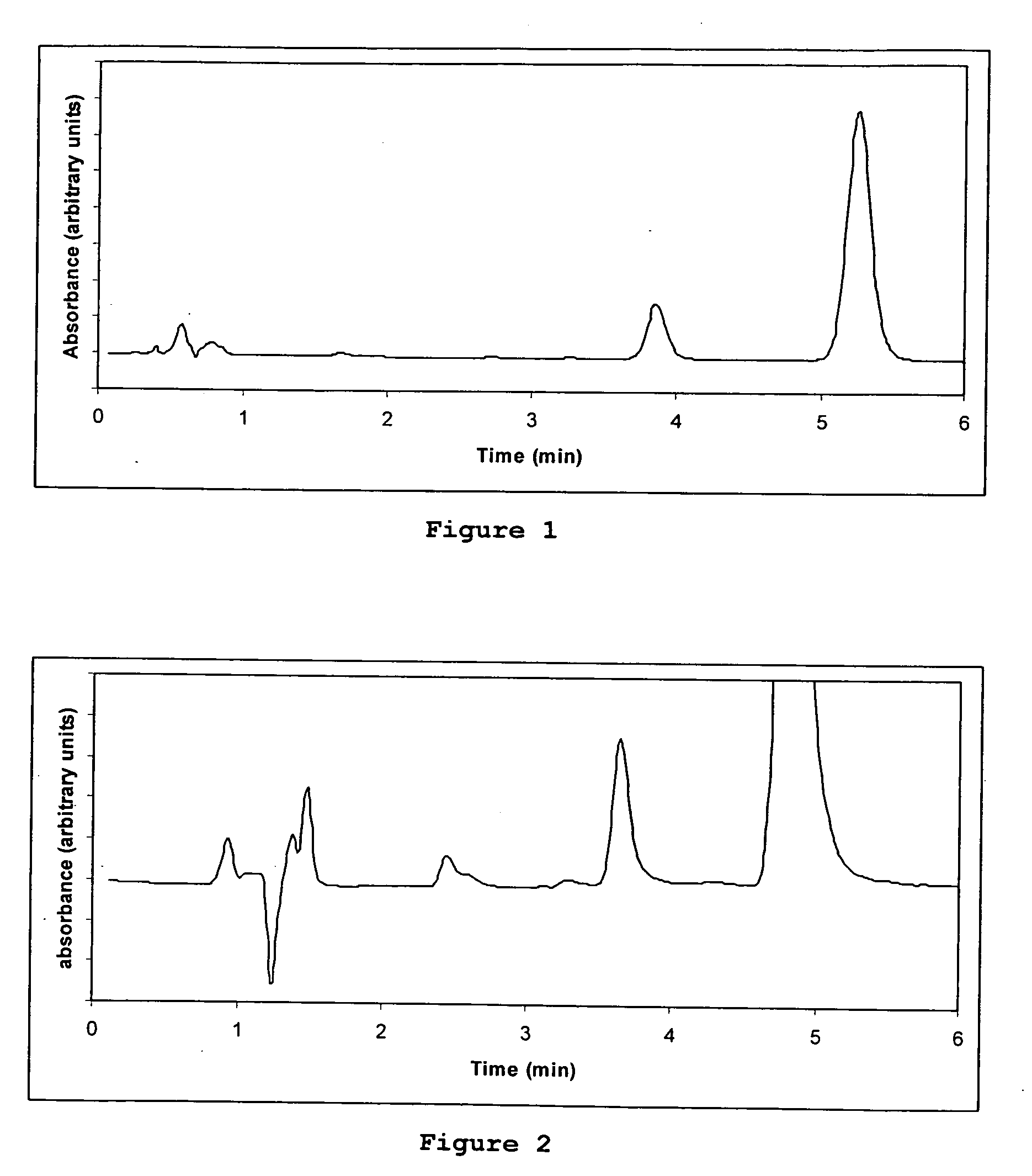
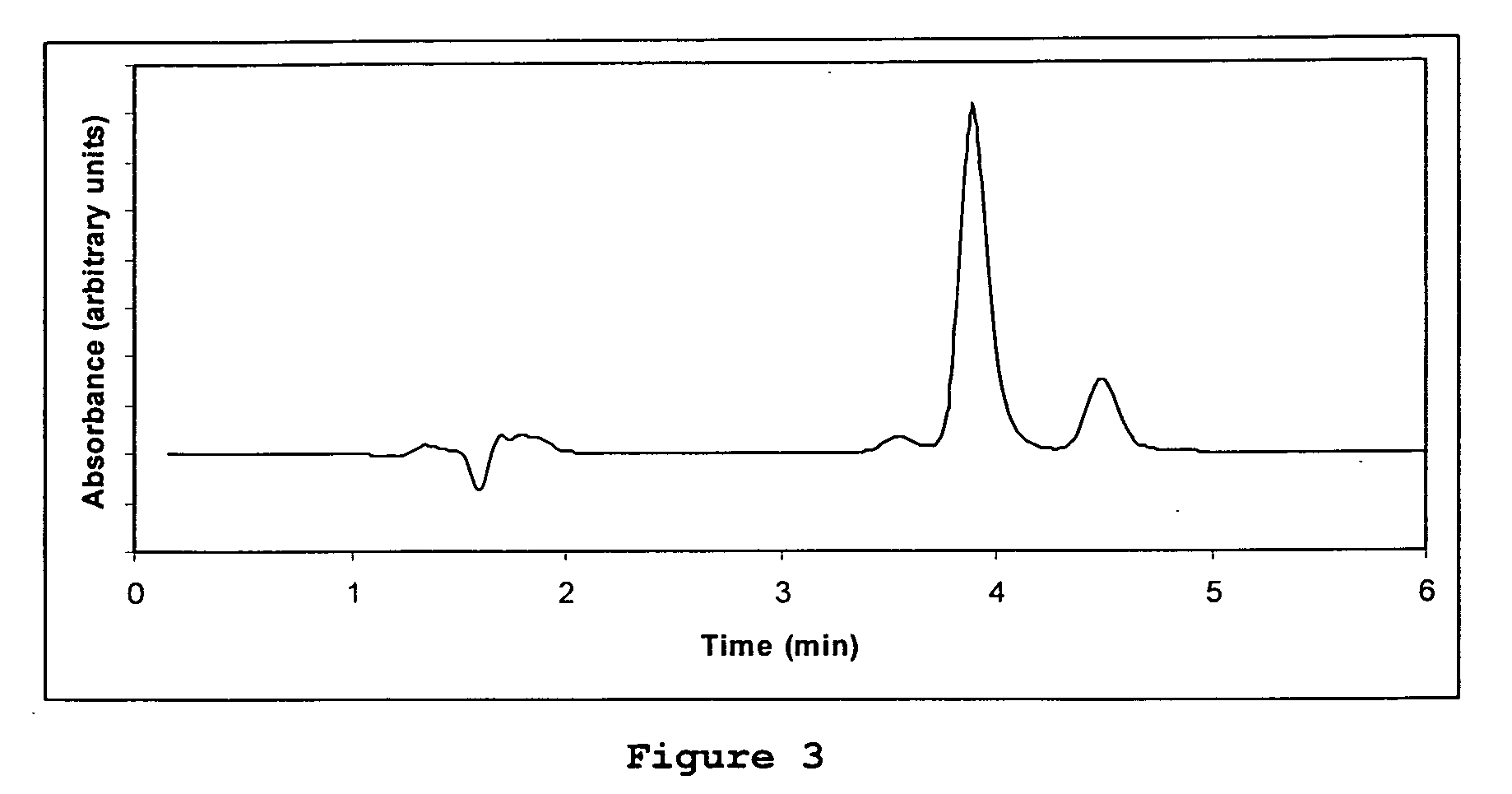
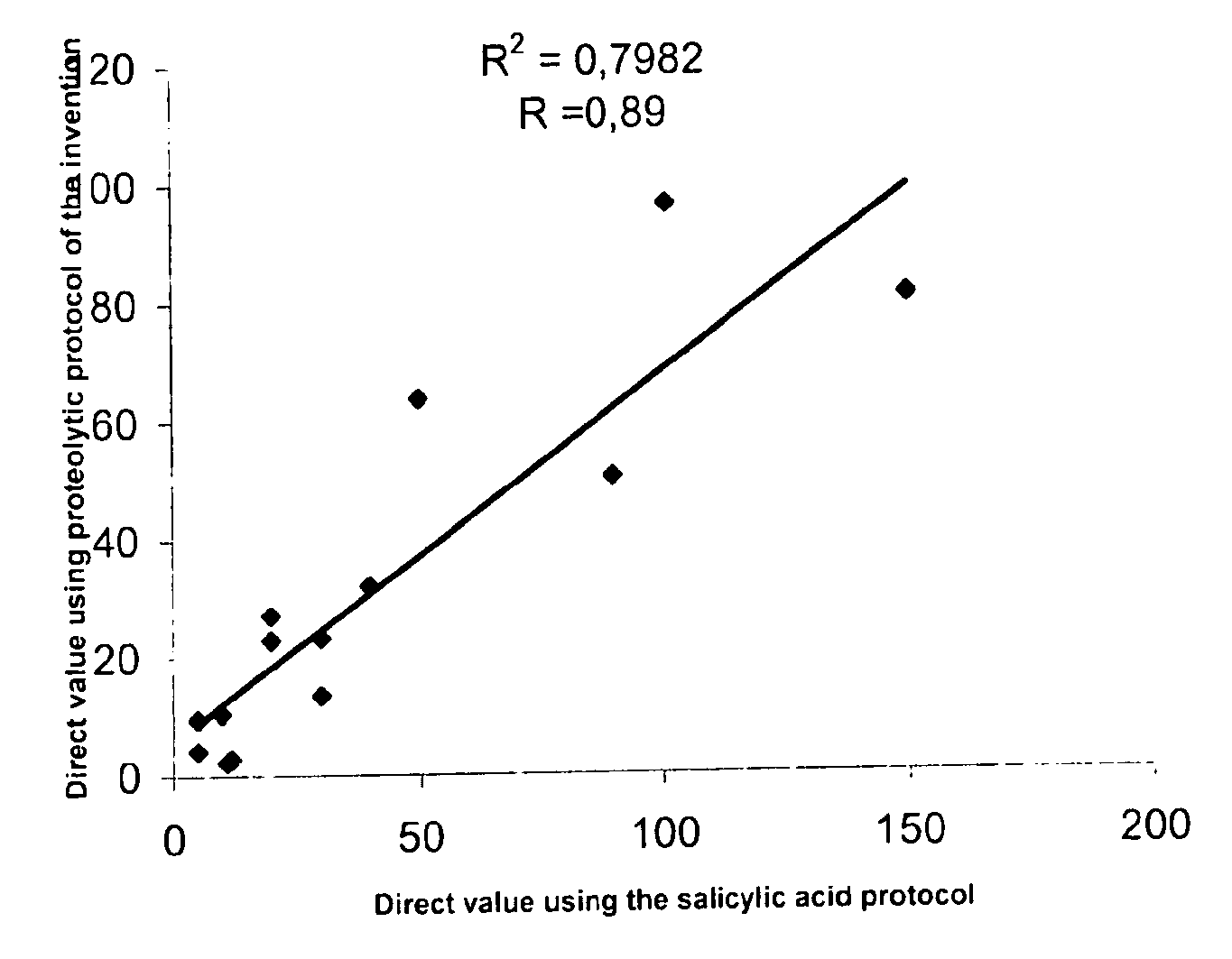
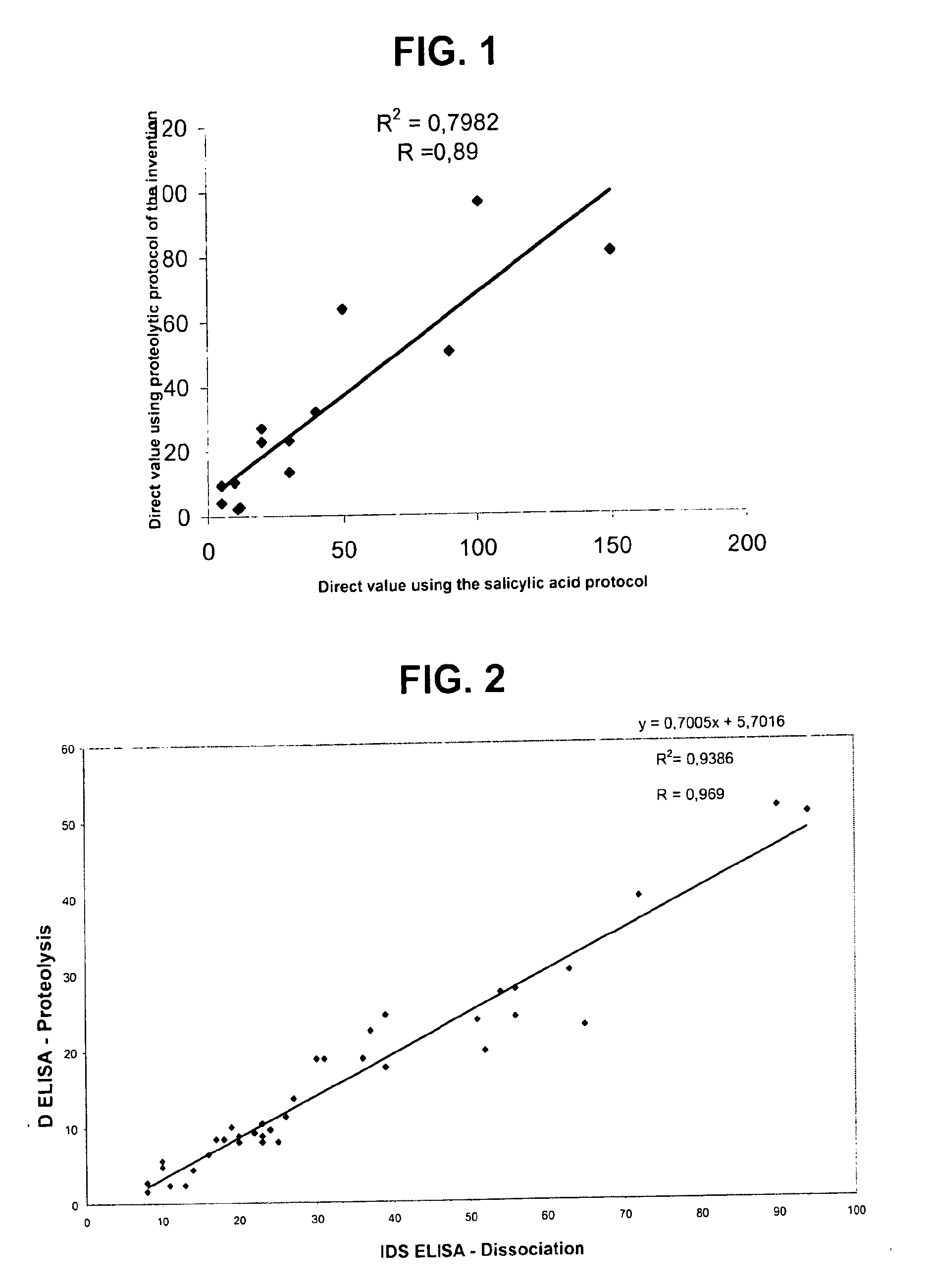
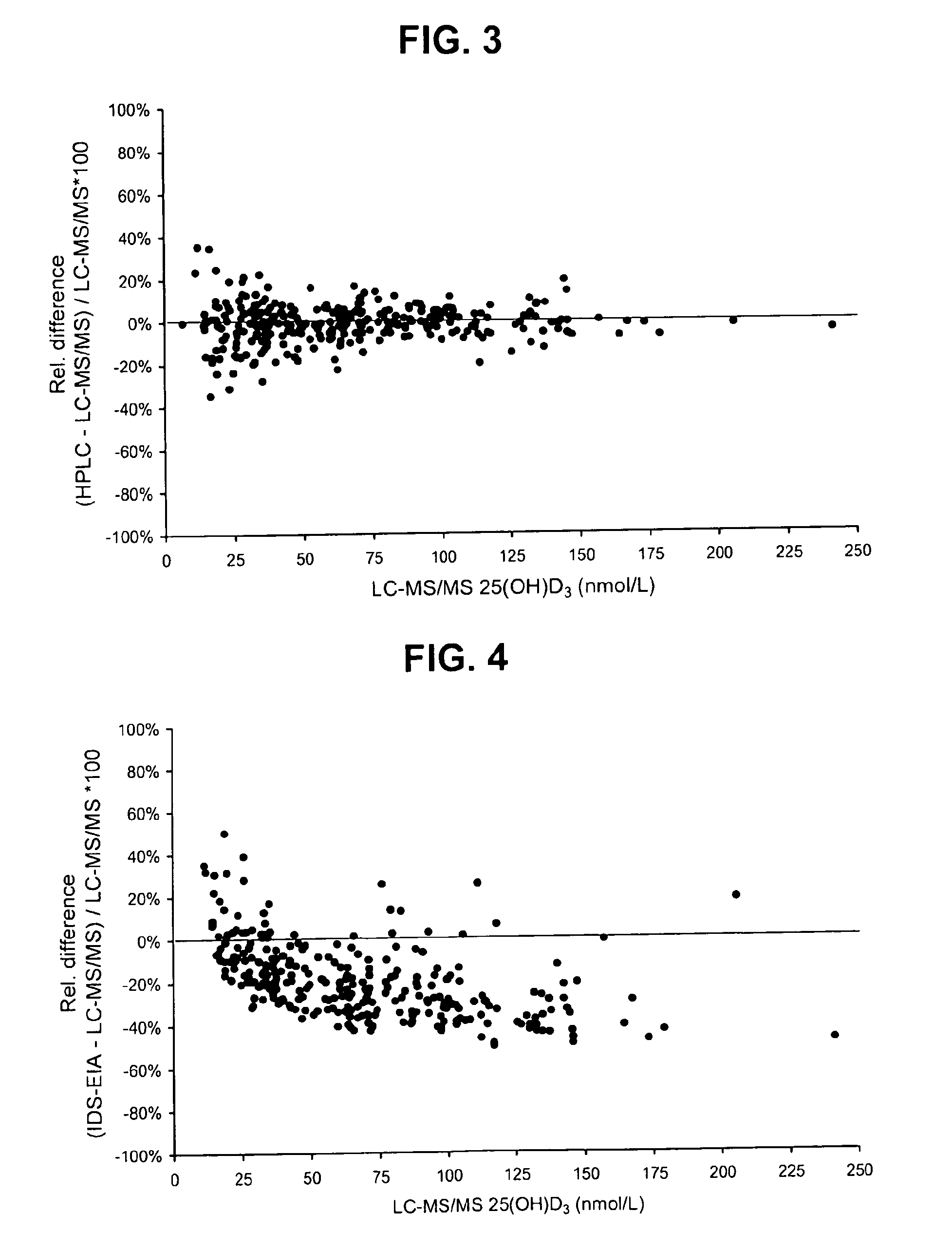
Direct determination of vitamin d in serum or plasma
ActiveUS20100068725A1Easy to controlPromote digestionBioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeCompetitive binding
A method for quantitating vitamin D metabolites directly in blood plasma or serum, without the need for prior purification of the vitamin D metabolites, comprising a digestion of the serum proteins with a serine protease such as proteinase K and sequence of steps for inhibiting the proteinase K activity in the competitive binding analysis. The advantages of this method are its high accuracy over the whole range of physiologically relevant values and that it can be easily adapted for a fully automated analysis of serum and plasma samples.
Owner:IMMUNDIAGNOSTIK
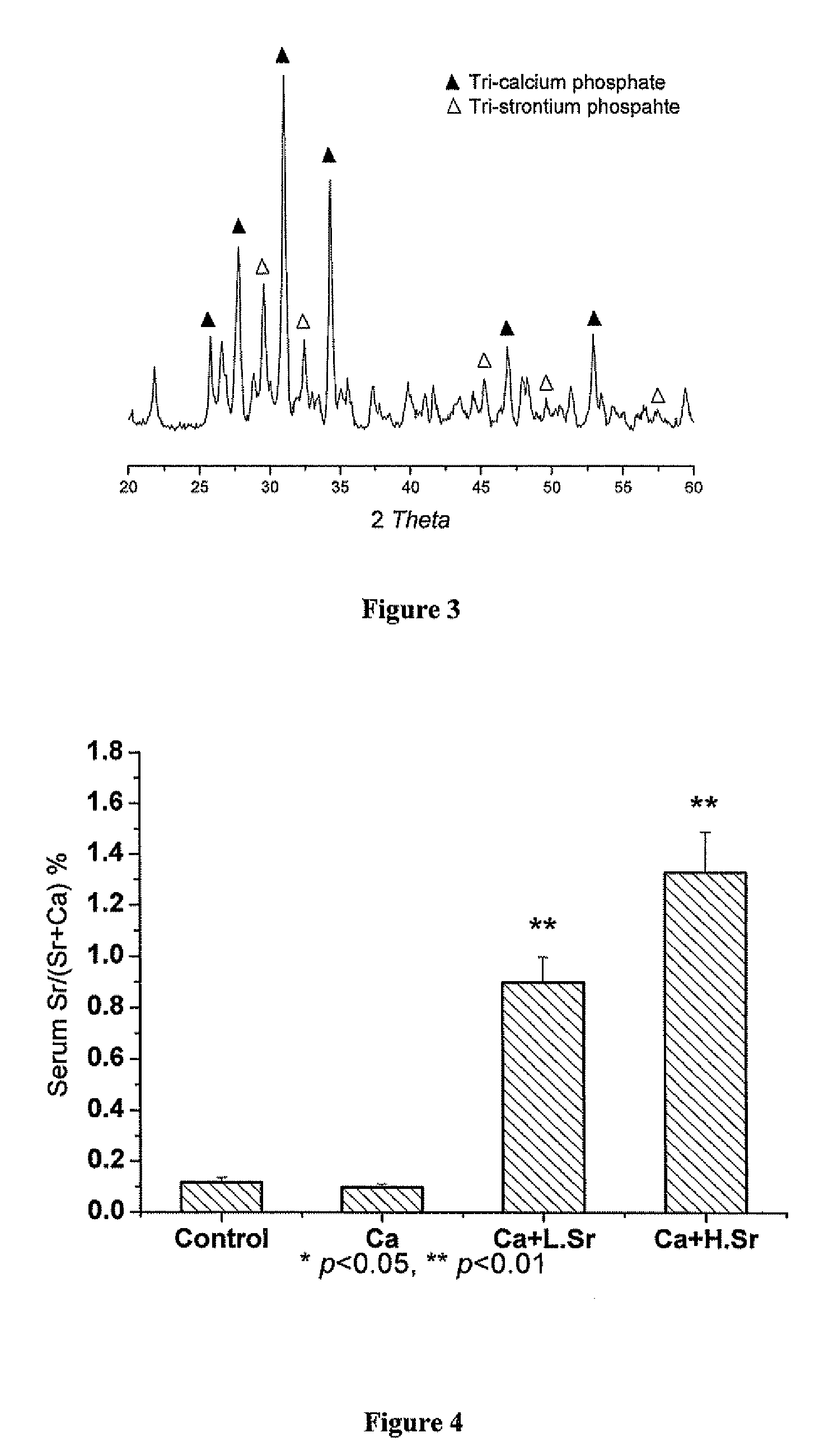
Strontium fortified calcium nano-and microparticle compositions and methods of making and using thereof
InactiveUS20080317807A1Good biocompatibilityPrevent looseningHeavy metal active ingredientsBiocideDiseasePhosphate
Compositions containing strontium fortified calcium nanoparticles and / or microparticles, and methods of making and using thereof are described herein. The strontium fortified calcium compounds contain calcium ions, calcium atoms, strontium ions, strontium atoms, and combinations thereof and one or more anions. Exemplary anions include, but are not limited to, citrate, phosphate, carbonate, and combinations thereof. The particles can be formulated for enteral or parenteral administration by incorporating the particles into a pharmaceutically carrier. The compositions can further contain one or more active agents useful for bone diseases or disorders, such as vitamin D, growth factors, and combinations thereof. The compositions can be used to treat or prevent one or more bone diseases or disorders of the bone, such as osteoporosis. Alternatively, the particles can be coated onto a substrate, such as the surface of an implant. The coatings can be used to improved biocompatibility of the implant, prevent loosening of the implant, reducing leaching of metal ions from metallic implants, and reduce corrosion. The coatings can be applied to the substrate using a variety of techniques well known in the art. In one embodiment, the coating is applied using electrophoretic deposition. The use of nano- and / or microparticles that provide high surface area helps to improve interfacial strength between the coating and the implant, which allows for the use of lower sintering temperatures. Lowering sintering temperatures minimizes or prevents thermal decomposition of the coating material and / or degradation of the implant material.
Owner:THE UNIVERSITY OF HONG KONG
Therapeutically effective 1alpha, 25-dihydroxyvitamin D3 analogs and methods for treatment of vitamin D diseases
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods of treating abnormal cell proliferation
A composition is described comprising a vitamin D analog and a retinoid wherein: (a) the vitamin D analog is capable of binding a vitamin D receptor or being converted in vivo into a compound capable of binding a vitamin D receptor; and (b) the retinoid is selected from the group consisting of retinol in a concentration of at least about 1.0% and a retinoid characterized by having a substitution at the 4-position. Further, methods of treating disorders characterized by abnormal cell-proliferation and / or cell-differentiation are also described.
Owner:LEO PHARMA PROD LTD AS LOVENS CHEM FAB PRODUKTIONS AS
24-sulfoximine vitamin D3 compounds
The present invention provides novel sulfoximine compounds, compositions comprising these compounds and methods of using these compounds as inhibitors of CYP24. In particular, the compounds of the invention are useful for treating diseases which benefit from a modulation of the levels of 1α,25-dihydroxy vitamin D3, for example, cell-proliferative disorders.
Owner:OPKO IRELAND GLOBAL HLDG LTD +1
Methods and compositions for disease treatment using inhalation
InactiveUS20120077786A1Increasing subject 's convenienceGood treatment complianceBiocideOrganic active ingredientsPulmonary edemaLung cancer
Methods and compositions for the treatment of pulmonary disease using inhalation are provided. In particular, the present disclosure provides novel methods and compositions for treating pulmonary diseases such as asthma, bronchitis, COPD, emphysema, lung cancer, pneumonia and pulmonary edema. In addition, the present disclosure provides novel methods and compositions for treating complications associated with pulmonary disease such as corticosteroid resistance and pulmonary tissue destruction. The compositions of the present disclosure comprise corticosteroid resistance agents including but not limited to vitamin D, calcitriol and equivalents thereof. The compositions of the present disclosure also comprise alveolar development and maintenance agents including but not limited to vitamin A, ATRA and equivalents thereof. The present invention provides effective administration of therapeutic agents to specific airways of the lungs by utilizing controlled site delivery.
Owner:MICRODOSE THERAPEUTX INC
Method of increasing bioavailability of alendronate or other bis-phosphonate by predose administration of vitamin D derivative
InactiveUS20050026871A1Improve bioavailabilityBiocidePhosphorous compound active ingredientsBioavailabilityVitamin D+Metabolites
The present invention relates to a method of increasing the bioavailability of a bis-phosphonate such as alendronate by administering an effective predose of a vitamin D derivative at least 6 hours before administering a therapeutic dose of the bis-phosphonate.
Owner:TEVA PHARM USA INC
Treating vitamin d insufficiency and deficiency with 25-hydroxyvitamin d2 and 25-hydroxyvitamin d3
ActiveUS20090311316A1Inhibit hepatic prohormone productionPromoteBiocideOrganic active ingredientsAdditive ingredientSustained Release Formulations
Owner:OPKO RENAL LLC
Method for preparing analogue of vitamin D
InactiveUS20070088007A1Reduce in quantityLow yieldOrganic active ingredientsBiocidePresent methodCompound (substance)
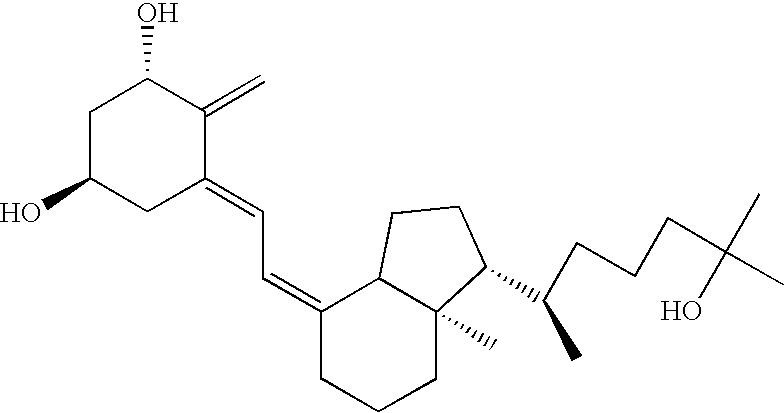
A method for preparing analogues of C1,C24-dihydroxy-vitamin D is disclosed. Especially the method for preparing calcipotriol and tacalcitol from a starting material of Vitamin D2 is disclosed here. Calcipotriol (compound 1(a)) and tacalcitol (compound 1(b)) can be synthesized by the method of the present invention. Moreover, only nine steps are needed for the synthesis of calcipotriol using the method. Likewise, only ten steps are needed for the synthesis of tacalcitol by the present method. Hence, the present method, with less process steps and higher yields, represents an improvement over the conventional methods.
Owner:FORMOSA LAB
Methods for detecting vitamin D metabolites
ActiveUS7745226B2Component separationMass spectrometric analysisMass Spectrometry-Mass SpectrometryVitamin D metabolism
Provided are methods of detecting the presence or amount of a vitamin D metabolite in a sample using mass spectrometry. The methods generally comprise ionizing a vitamin D metabolite in a sample and detecting the amount of the ion to determine the presence or amount of the vitamin D metabolite in the sample. Also provided are methods to detect the presence or amount of two or more vitamin D metabolites in a single assay.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
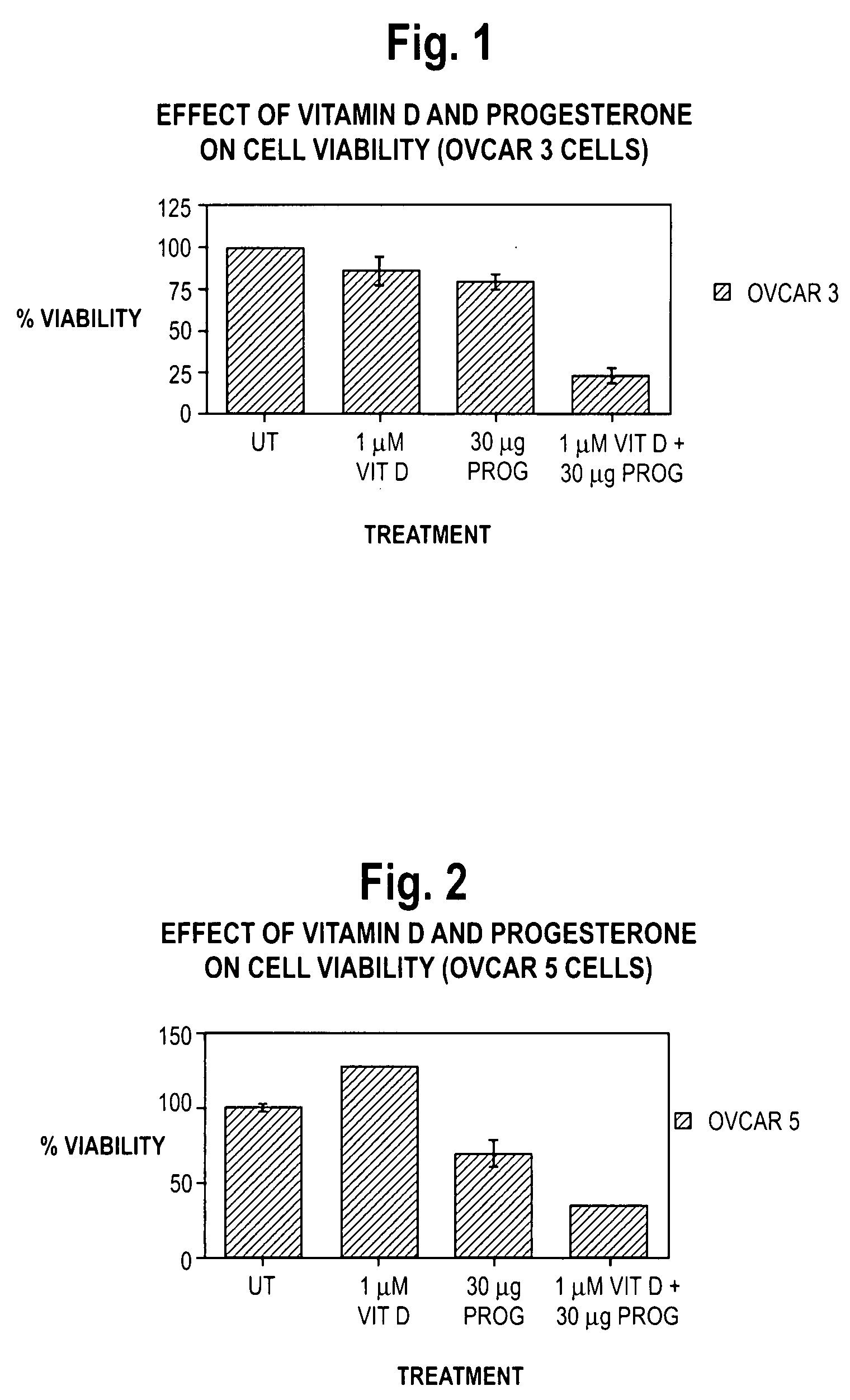
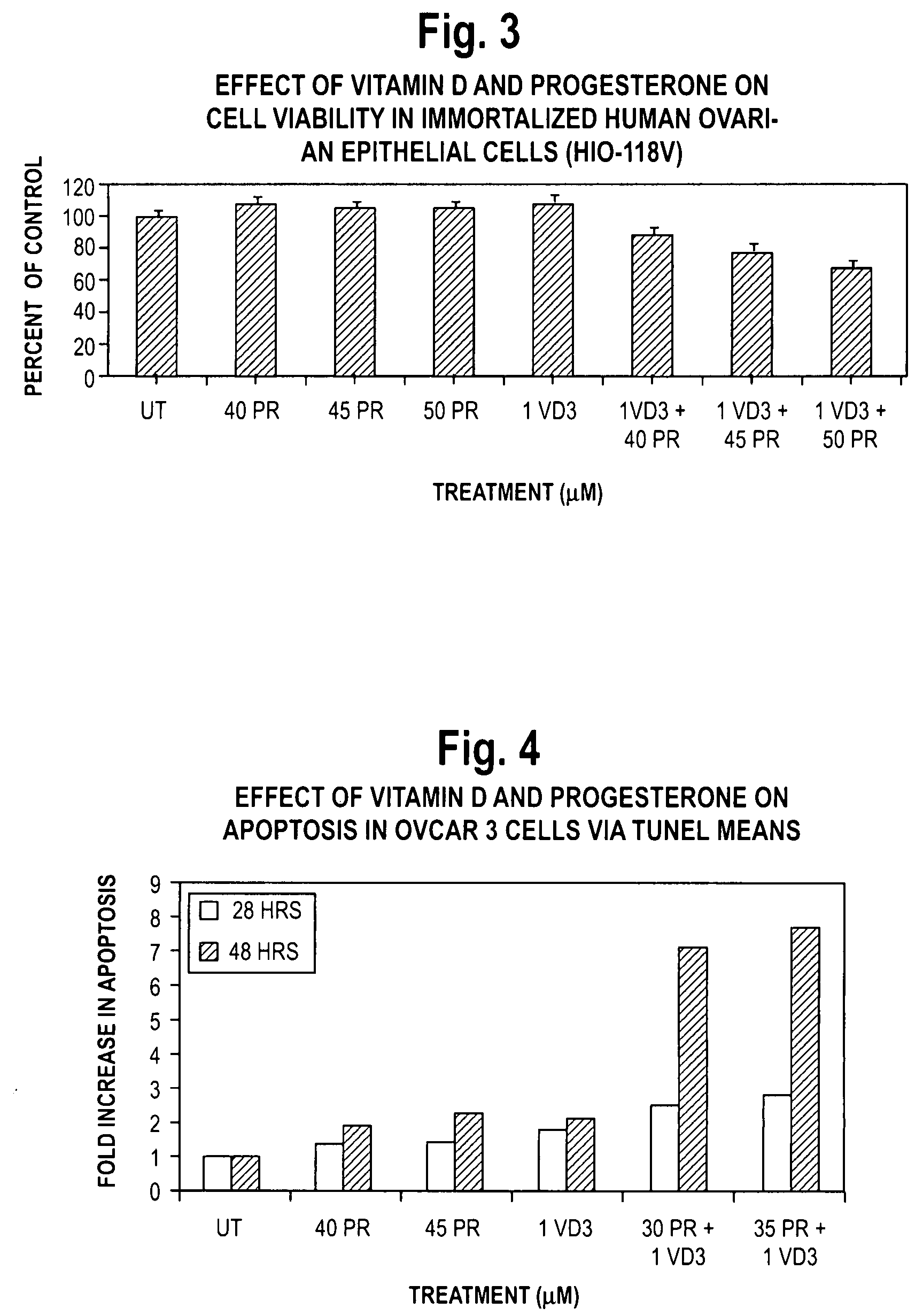
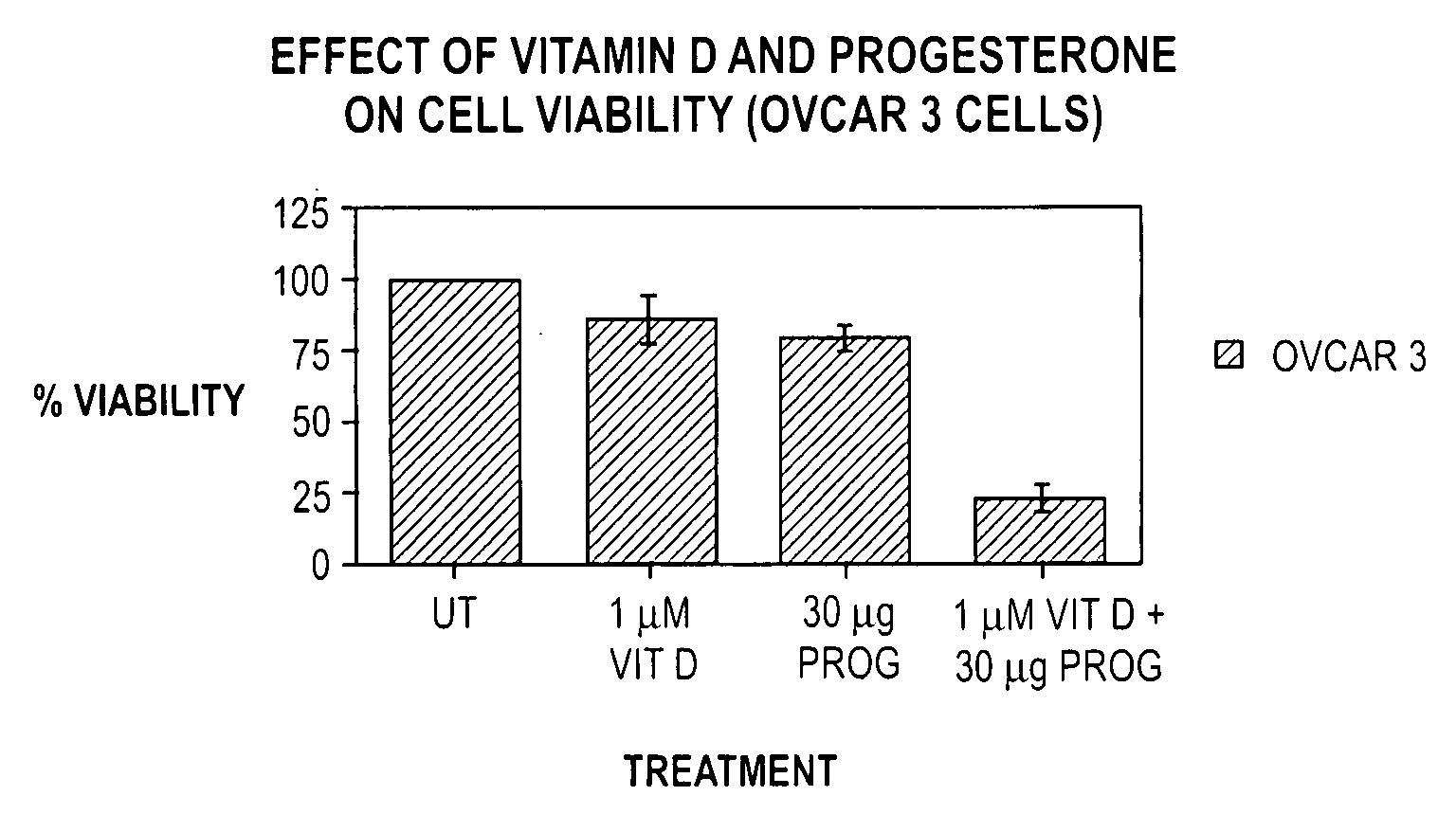
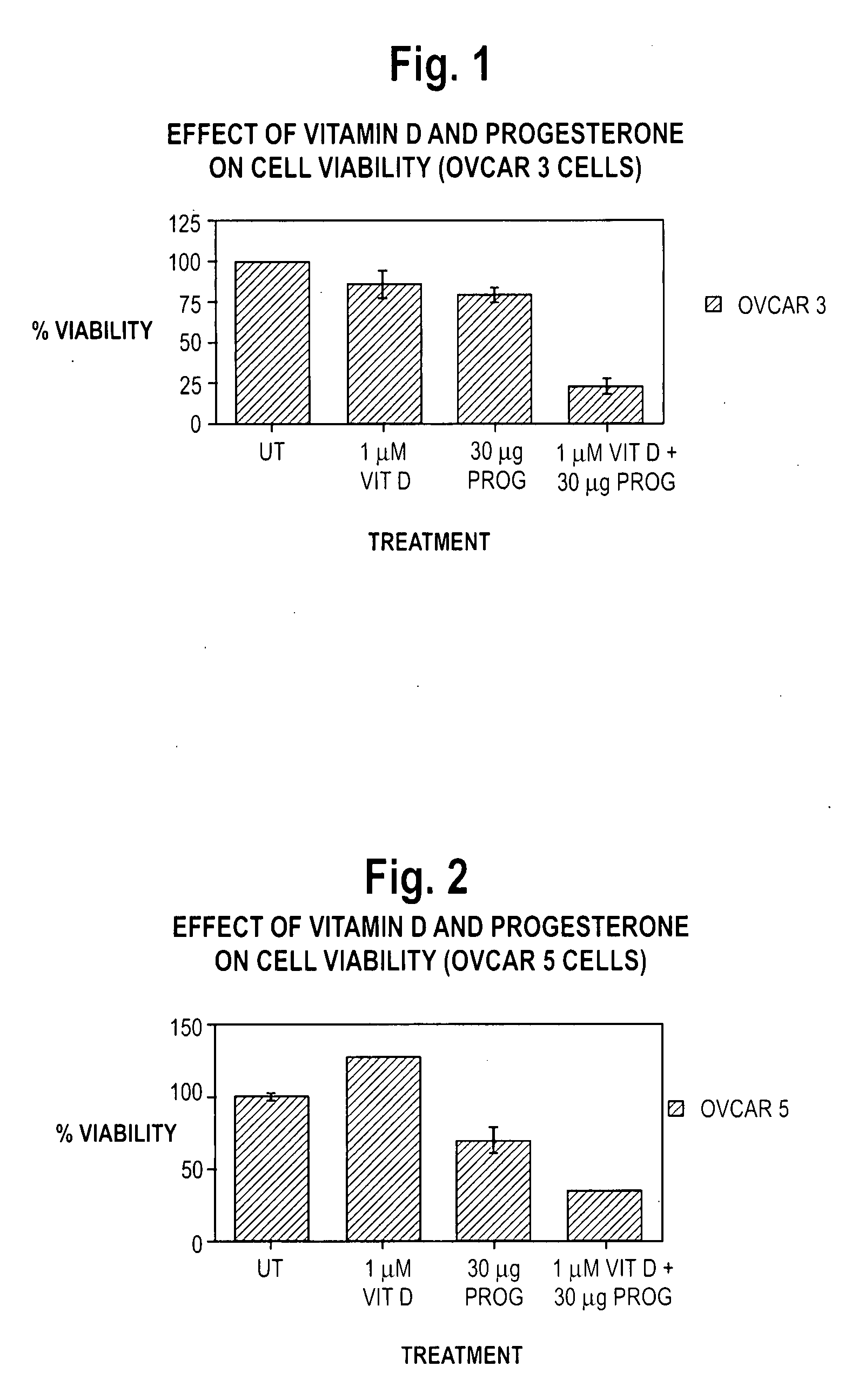
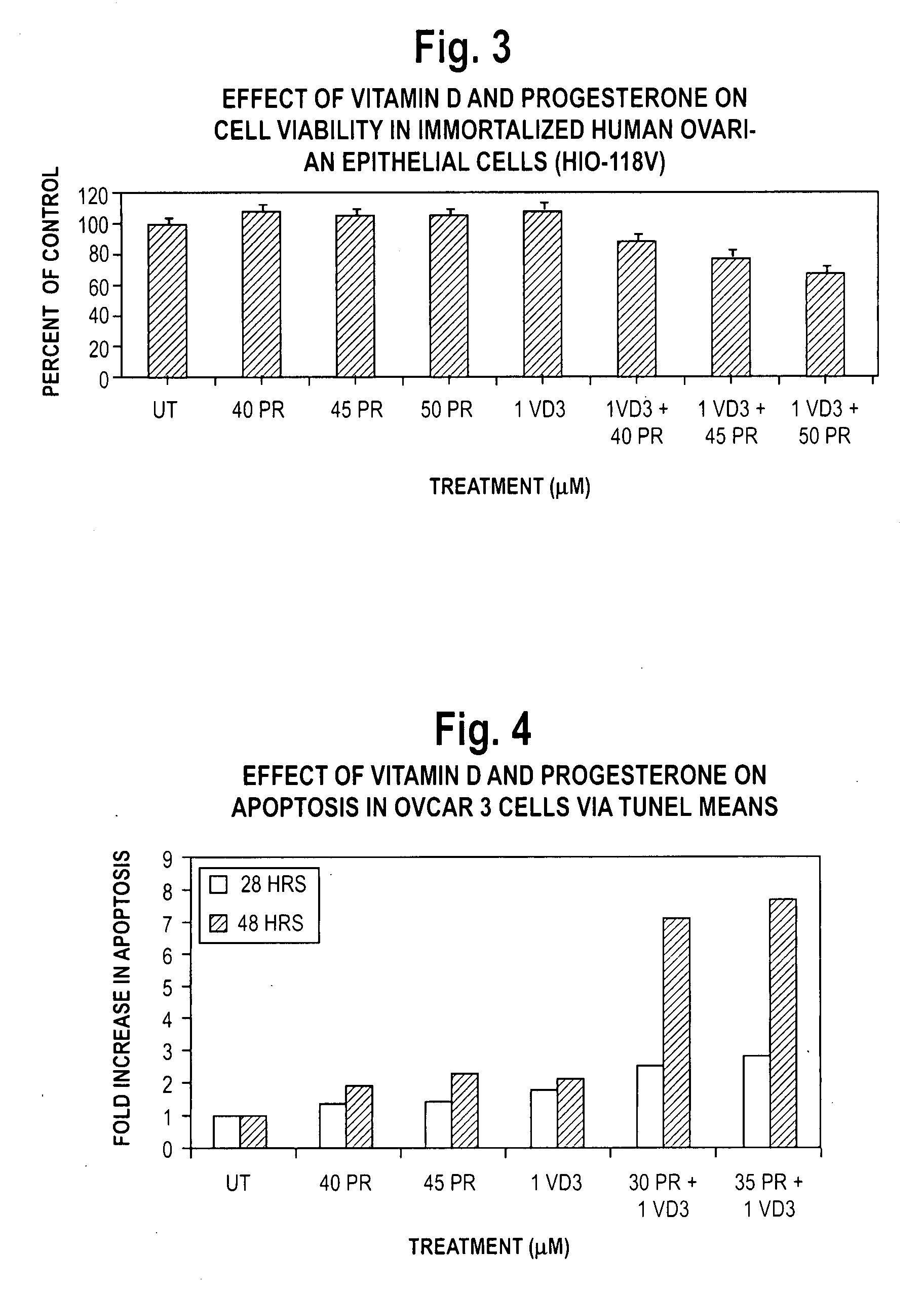
Pharmaceutical products containing hormones and a 25-hydroxy vitamin d compound
InactiveUS20080312198A1Maximum safetyGood effectOrganic active ingredientsBiocideMedicineProgesterone agent
The present invention relates to pharmaceutical products containing progestins in combination with the 25 hydroxy Vitamin D compounds. The 25 hydroxy Vitamin D compounds are preferably administered with the progestins. In OC and HRT regimens, the 25 hydroxy Vitamin D compounds can be administered daily, or on a non-daily basis, and if so, preferably when the progestin dosages are the highest in the cycle.
Owner:RODRIGUEZ GUSTAVO C
Pharmaceutical products containing hormones and a 25-hydroxy vitamin d compound
InactiveUS20080312197A1Maximum safetyGood effectBiocideOrganic active ingredientsMedicineVitamin D+Metabolites
The present invention relates to pharmaceutical products containing progestins in combination with the 25 hydroxy Vitamin D compounds. The 25 hydroxy Vitamin D compounds are preferably administered with the progestins. In OC and HRT regimens, the 25 hydroxy Vitamin D compounds can be administered daily, or on a non-daily basis, and if so, preferably when the progestin dosages are the highest in the cycle.
Owner:RODRIGUEZ GUSTAVO C
Vitamin d forming sunscreen
ActiveUS20110268678A1Promoting tanningProtecting skin from damageAntibacterial agentsCosmetic preparationsDiseaseUltraviolet
A composition suitable for providing protection against ultraviolet radiation containing one or more active ultraviolet radiation absorber through which ultraviolet radiation in approximately the 295 to 315 nanometer range is permitted to enter the skin in an amount sufficient for the body to produce a healthful and disease-opposing quantity of vitamin D3 and chemical precursors thereof. The composition is particularly used in sunscreens promoting on the one hand vitamin D3 production, on the other hand protecting the skin against harmful ultraviolet radiation.
Owner:ARMSTRONG ERNEST T
Compositions, kits and methods for nutrition supplementation
The present invention relates to compositions, kits and methods for the administration of various vitamin, mineral and nutrient compositions, and in a specific embodiment, the compositions, kits and methods may utilize or include vitamin D, iodine, vitamin B1, vitamin B6, vitamin B12, vitamin B2, vitamin B9, vitamin B3, vitamin E, vitamin A, vitamin C, iron, zinc, copper, magnesium, omega 3 fatty acids and one or more pharmaceutically acceptable carriers.
Owner:EXELTIS FRANCE
Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3
ActiveUS8426391B2Inhibit hepatic prohormone productionPromoteBiocideOrganic active ingredientsSustained Release FormulationsVitamin D+Metabolites
Owner:OPKO RENAL LLC
Refrigeration-shelf-stable ultra-pasteurized or pasteurized infant formula
InactiveUS6039985AMaintain qualityReduce degradationSugar food ingredientsVitamin food ingredientsPantothenic acidVitamin B6 synthesis
Refrigeration-shelf-stable ready-to-feed and concentrated infant formulas prepared through an ultra-pasteurization and / or pasteurization process, comprise per five fluid ounces from about 1.8 to about 6.3 grams of protein; from about 3.3 to about 15.9 grams of fat; from about 300 mg to about 3000 mg of linoleic acid; from about 250 to about 900 IU of Vitamin A; from about 40 to about 180 IU of Vitamin D; from about 0.7 to about 9 IU of Vitamin E; from about 4 to about 24 mcg of Vitamin K; from about 40 to about 300 mcg of Thiamine (Vitamin B1); from about 60 to about 450 mcg of Riboflavin (Vitamin B2); from about 35 to about 180 mcg of Vitamin B6; from about 0.15 to about 0.9 mcg of Vitamin B12; from about 250 to about 3150 mcg of Niacin; from about 4 to about 48 mcg of Folic Acid (Folacin); from about 300 to about 1500 mcg of Pantothenic Acid; from about 1.5 to about 13.2 mcg of Biotin; from about 8 to about 36 mg of Vitamin C (Ascorbic Acid); from about 7 to about 48 mg of Choline; from about 4 to about 18 mg of Inositol; from about 60 to about 234 mg of Calcium; from about 30 to about 159 mg of Phosphorus; from about 6 to about 24 mg of Magnesium; from about 0.15 to about 5.4 mg of Iron; from about 0.5 to about 3 mg of Zinc; from about 5 to about 45 mcg of Manganese; from about 60 to about 270 mcg of Copper; from about 5 to about 75 mcg of Iodine; from about 20 to about 81 mg of Sodium; from about 80 to about 324 mg of Potassium; and from about 55 to about 195 mg of Chloride; wherein the total caloric content is from about 80 kilocalories to about 300 kilocalories per five fluid ounces.
Owner:KAMAREI A REZA +1
Therapeutically effective 1 alpha ,25-dihydroxyvitamin D3 analogs and methods for treatment of vitamin D diseases
A method for treatment of diseases caused by deficiency or overproduction of the vitamin D3 metabolites by administering analogs of 1 alpha ,25-dihydroxyvitamin D3. These analogs are selective agonists or antagonists for the genomic and rapid nongenomic cellular responses. A pharmaceutical composition comprising 1 alpha ,25-dihydroxyvitamin D3 analog.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com