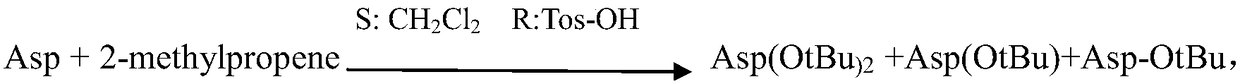A kind of preparation method of aspartic acid-1-tert-butyl ester derivative
A technology of aspartic acid and tert-butyl acetate, which is applied in the preparation of carbamic acid derivatives, organic compounds, cyanide reaction, etc., can solve the problems of high cost, expensive hydrogenolysis, and long lines, and achieve production The effect of high timeliness, short steps and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a kind of preparation method of aspartic acid-1-tert-butyl ester derivative, comprising the following steps:
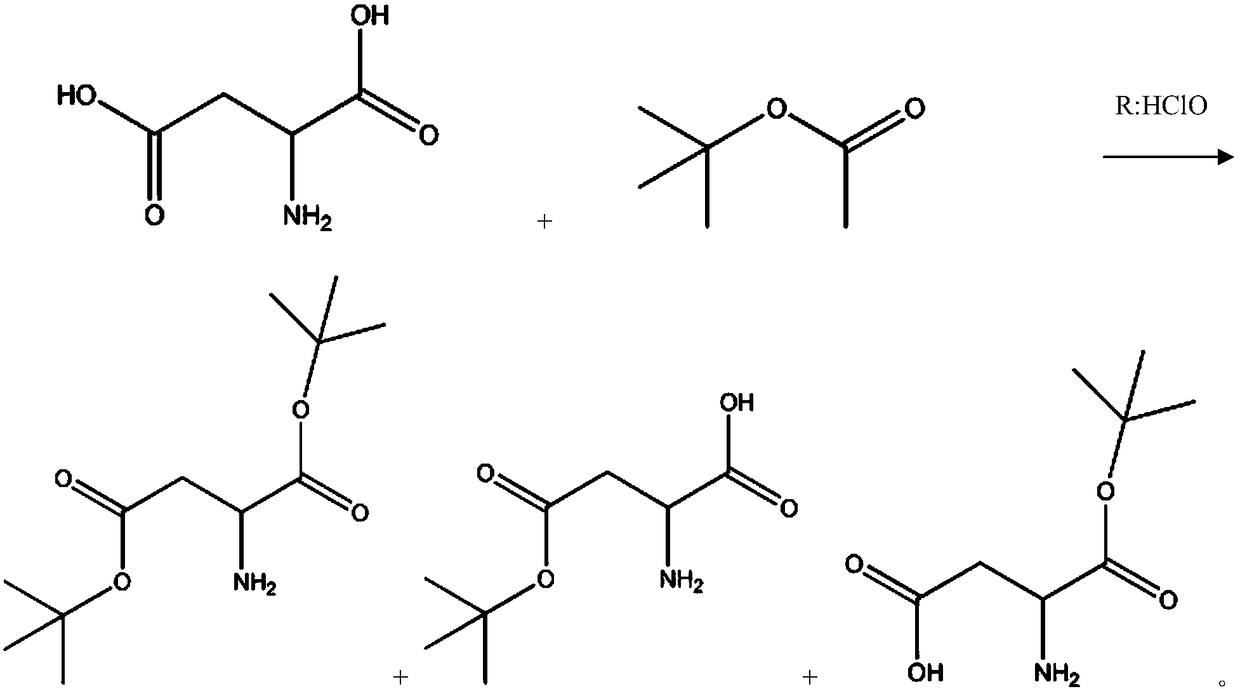
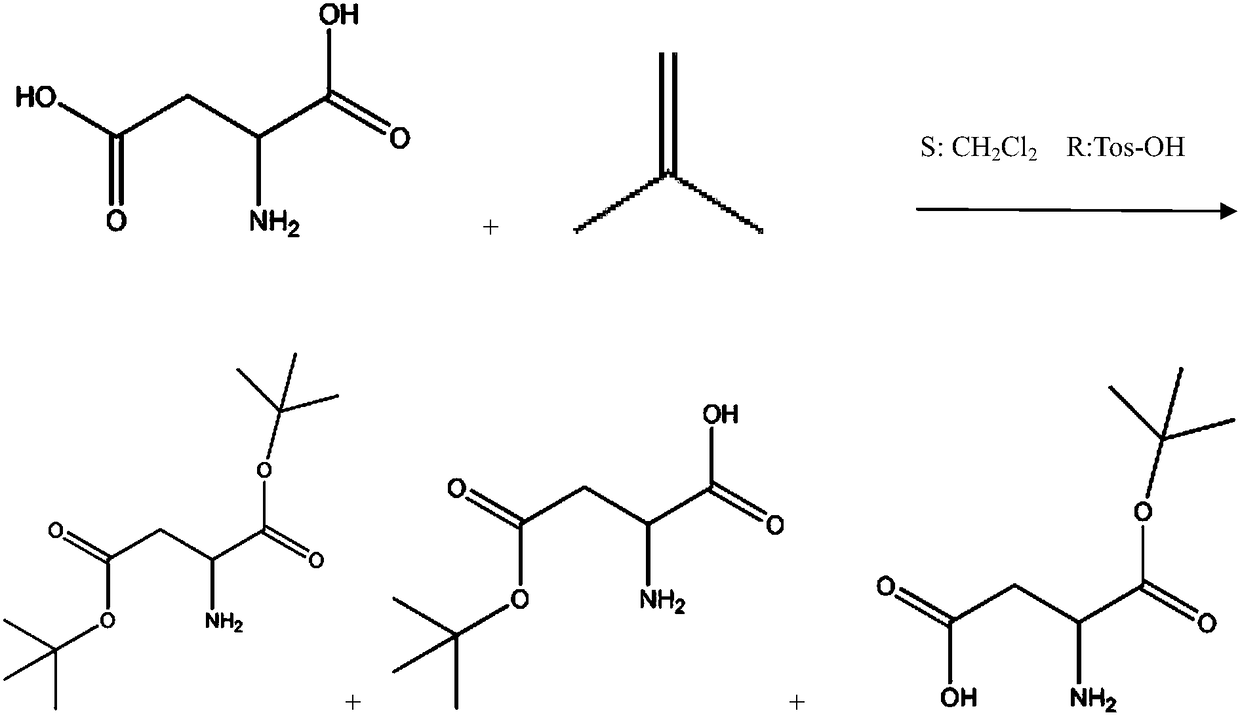
[0029] (1) Aspartic acid (Asp) is prepared aspartic acid-4-tert-butyl ester (Asp(OtBu)) and aspartic acid-1-tert-butyl ester (Asp-OtBu) mixture;
[0030] (2) Mix aspartic acid-4-tert-butyl ester (Asp(OtBu)) and aspartic acid-1-tert-butyl ester (Asp-OtBu) mixture with transition metal M salt to obtain M[Asp( OtBu)] x and M(Asp-OtBu) x A mixture of , where 1≤x≤2;
[0031] (3) The mixture obtained in step (2) reacts with a protective reagent again, and selectively reacts to obtain a derivative of aspartic acid-1-tert-butyl ester;
[0032] The selective reaction process is:
[0033] Regulation M[Asp(OtBu)] x and M(Asp-OtBu) x The pH value of the mixture solution is 8-9, adding a protective reagent, maintaining the pH value of the reaction solution at 8-9, reacting for 7-10 hours, and then acidifying, extracting, crystallizing, f...
Embodiment 1
[0060] The preparation of embodiment 1 fluorenyl moxycarbonyl aspartic acid-1-tert-butyl ester
[0061] Specifically include the following steps:
[0062] (1) Prepare aspartic acid-4-tert-butyl ester (Asp(OtBu)) and aspartic acid-1-tert-butyl ester (Asp-OtBu) mixture from aspartic acid (Asp): three ports in 2000mL Add 581g of tert-butyl acetate and 133g of aspartic acid into the bottle, stir, add 100mL of perchloric acid dropwise, react at 20°C for 48 hours, cool down to 0°C, add 600mL of water, and use Na 2 CO 3 Neutralize to pH=8~9, separate liquid, then use 100mL 1% Na 2 CO 3 The aqueous solution was washed three times, the aqueous phase was combined, and extracted three times with 200 ml of petroleum ether;
[0063] (2) Transfer the aqueous phase obtained in step (1) into a 2L three-necked flask, and then add 187.5g CuSO 4 ·5H 2 O, stir, and adjust the pH to 8-9 with sodium carbonate. Add 100ml of tetrahydrofuran, add 33.7g of Fmoc-OSu, maintain the pH at 8-9, and r...
Embodiment 2
[0065] The preparation of embodiment 2 fluorenyl methaneoxycarbonyl aspartic acid-1-tert-butyl ester
[0066] Specifically include the following steps:
[0067] (1) Prepare aspartic acid-4-tert-butyl ester (Asp(OtBu)) and aspartic acid-1-tert-butyl ester (Asp-OtBu) mixture from aspartic acid (Asp): three ports in 3000mL Add 1162g of tert-butyl acetate and 133g of aspartic acid into the bottle, stir, add 130mL of perchloric acid dropwise, react at 15°C for 30 hours, cool down to 0°C, add 600mL of water, and use Na 2 CO 3 Neutralize to pH=8~9, separate liquid, then use 100mL 1% Na 2 CO 3 The aqueous solution was washed three times, the aqueous phase was combined, and extracted three times with 200 ml of petroleum ether;
[0068] (2) Transfer the aqueous phase obtained in step (1) into a 2L three-necked flask, and then add 93.75g CuSO 4 ·5H 2 O, stir, and adjust the pH to 8-9 with sodium carbonate. Add 100ml of tetrahydrofuran and 67.4g of Fmoc-OSu, maintain the pH at 8-9,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com