A kind of preparation method of glutamic acid-1-tert-butyl ester derivative
A technology of tert-butyl acetate and glutamic acid is applied in the preparation of carbamic acid derivatives, the preparation of organic compounds, the preparation of cyanide reactions, etc. The effect of high production time, short steps and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a preparation method of glutamic acid-1-tert-butyl ester derivatives, comprising the following steps:
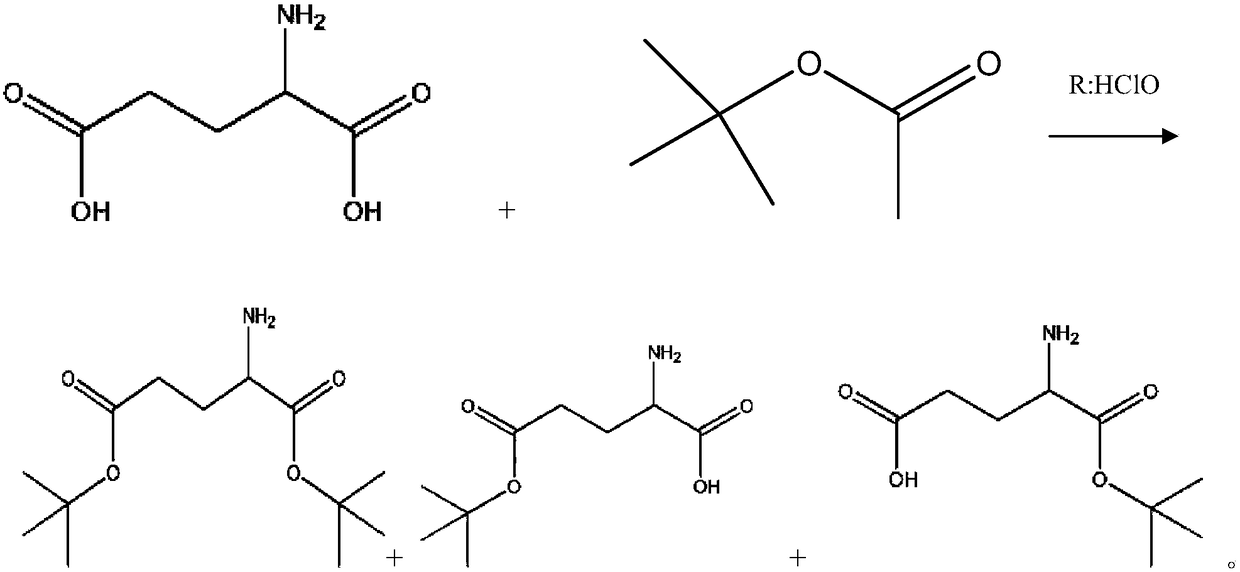
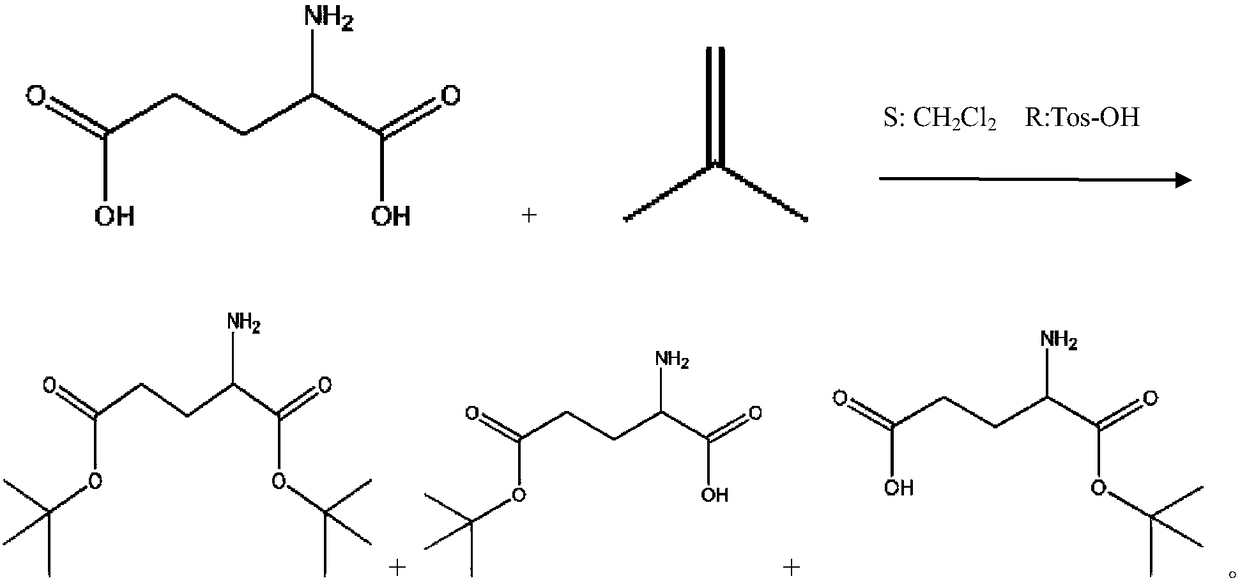
[0028] (1) Prepare glutamic acid-5-tert-butyl ester (Glu(OtBu)) and glutamic acid-1-tert-butyl ester (Glu-OtBu) mixture from glutamic acid (Glu);
[0029] (2) Mix glutamic acid-5-tert-butyl ester (Glu(OtBu)) and glutamic acid-1-tert-butyl ester (Glu-OtBu) mixture with transition metal M salt to obtain M[Glu(OtBu) ] x and M(Glu-OtBu) x A mixture of , where 1≤x≤2;
[0030] (3) Step (2) gained mixture reacts with protection reagent again, and selective reaction obtains the derivative of glutamate-1-tert-butyl ester; Protection reagent is FmocOSu, Fmoc-Cl, (Boc) 2 O, CbzOSu or Cbz-Cl;
[0031] The selective reaction process is:
[0032] Regulated M[Glu(OtBu)] x and M(Glu-OtBu) x The pH value of the mixture solution is 8 to 9, adding a protective reagent, maintaining the pH value of the reaction solution at 8 to 9, reacting for 7 to 10 hours, ...
Embodiment 1
[0059] The preparation of embodiment 1 fluorenyl methaneoxycarbonyl glutamic acid-1-tert-butyl ester
[0060] Specifically include the following steps:
[0061] (1) Prepare glutamic acid-5-tert-butyl ester (Glu(OtBu)) and glutamic acid-1-tert-butyl ester (Glu-OtBu) mixture from glutamic acid (Glu): add 581g of tert-butyl acetate and 147g of glutamic acid were stirred, and 100mL of perchloric acid was added dropwise, reacted at 20°C for 48 hours, cooled to 0°C, and then added with 600mL of water, washed with Na 2 CO 3 Neutralize to pH=8~9, separate liquid, then use 100mL 1% Na 2 CO 3 The aqueous solution was washed three times, the aqueous phase was combined, and extracted three times with 200 ml of petroleum ether;
[0062] (2) Transfer the aqueous phase obtained in step (1) into a 2L three-necked flask, and then add 187.5g CuSO 4 ·5H 2 O, stir, and adjust the pH to 8-9 with sodium carbonate. Add 100ml of tetrahydrofuran, add 33.7g of Fmoc-OSu, maintain the pH at 8-9, a...
Embodiment 2
[0064] The preparation of embodiment 2 fluorenyl methaneoxycarbonyl glutamate-1-tert-butyl ester
[0065] Specifically include the following steps:
[0066] (1) Prepare glutamic acid-5-tert-butyl ester (Glu(OtBu)) and glutamic acid-1-tert-butyl ester (Glu-OtBu) mixture from glutamic acid (Glu): add 1162g tert-butyl acetate and 147g glutamic acid, stir, add dropwise 130mL perchloric acid, react at 15°C for 30 hours, cool down to 0°C, add 600mL water, use Na 2 CO 3 Neutralize to pH=8~9, separate liquid, then use 100mL 1% Na 2 CO 3 The aqueous solution was washed three times, the aqueous phase was combined, and extracted three times with 200 ml of petroleum ether;
[0067] (2) Transfer the aqueous phase obtained in step (1) into a 2L three-necked flask, and then add 93.75g CuSO 4 ·5H 2 O, stir, and adjust the pH to 8-9 with sodium carbonate. Add 100 ml of tetrahydrofuran, 67.4 g of Fmoc-OSu, maintain the pH at 8-9, and react for 8 hours to obtain the crude product fluoreny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com