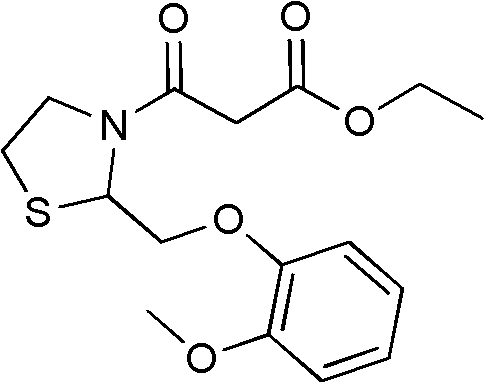
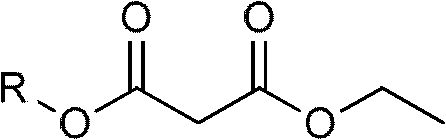
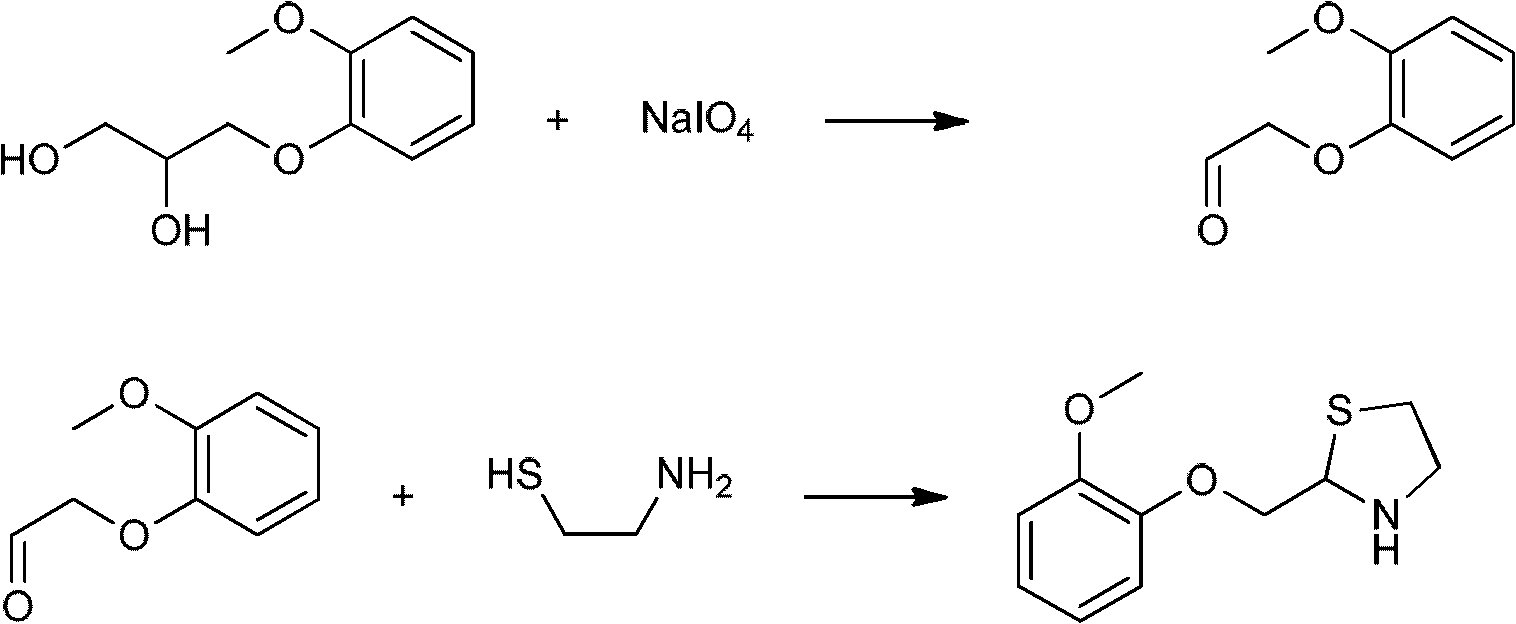
Moguisteine synthesis process
A technology of synthesis process and process steps, applied in the field of medicine, achieves the effects of easy operation, simple equipment and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, prepare mogisteine according to the following processing steps:
[0031] (1) Acyl chloride synthesis reaction generates monoethyl malonate acid chloride: add 26.42 g (0.2 mol) of monoethyl malonate in a 500 ml flask, add 100 ml of dichloromethane, stir in an ice bath for 10 minutes, and drop from the dropping funnel Add 17ml (0.24mol) of thionyl chloride, after the dropwise addition, continue to stir for 10 minutes, then heat and reflux for 1 hour, then cool and stir in an ice bath for 10 minutes, add 100ml of water, stir, and separate the liquids to obtain light brown malonic acid Dichloromethane solution of monoethyl chloride;
[0032] (2) preparation of dichloromethane solution of monoethyl malonate acid chloride
[0033] Add 34.05 g (0.2 mol) of potassium monoethyl malonate to a 500 ml flask, add 100 ml of dichloromethane, stir in an ice bath for 10 minutes, add 17 ml (0.24 mol) of thionyl chloride dropwise from the dropping funnel, and the addition...
Embodiment 2
[0037] Embodiment 2, by the operation method of step 4 in embodiment 1, use the dichloromethane solution of the malonate monoethyl chloride prepared in step 2 in embodiment 1, obtain mogistein white powder 27.8g, yield is according to Guaifenesin 82%. The melting point is 73.2-74.5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com