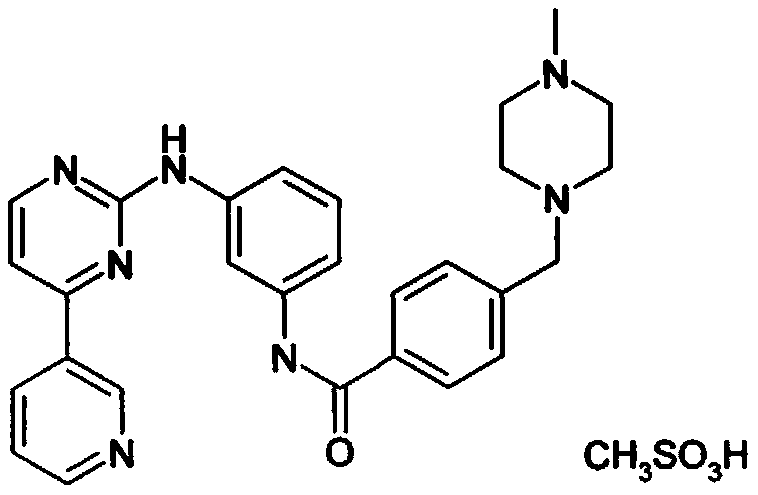
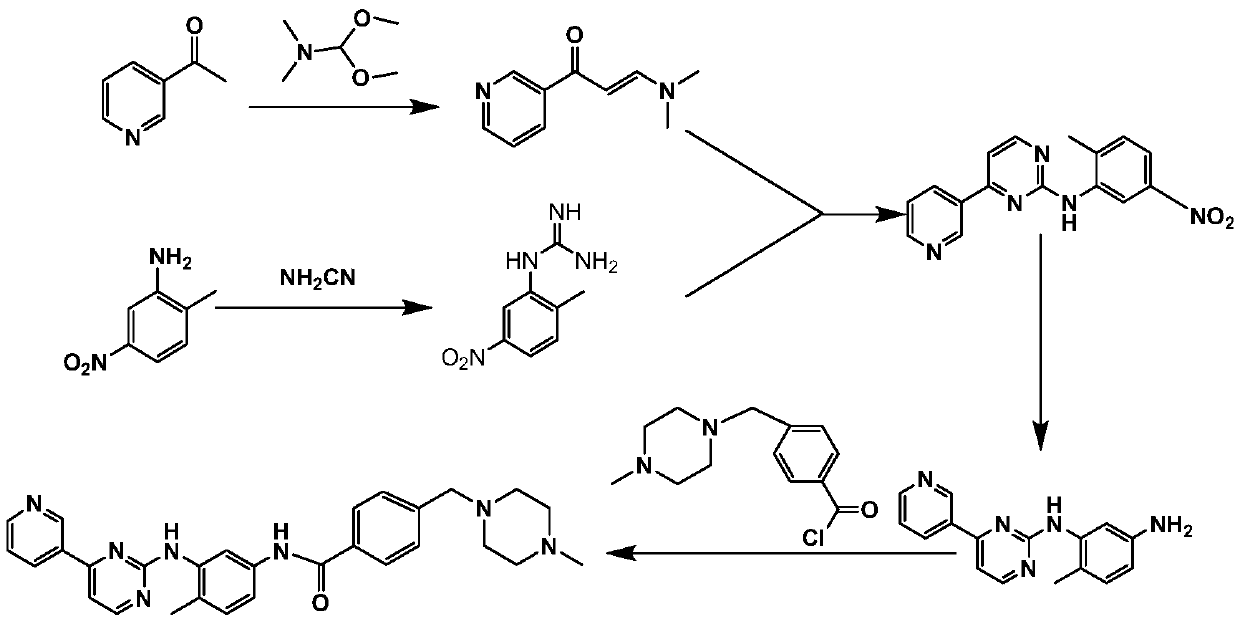
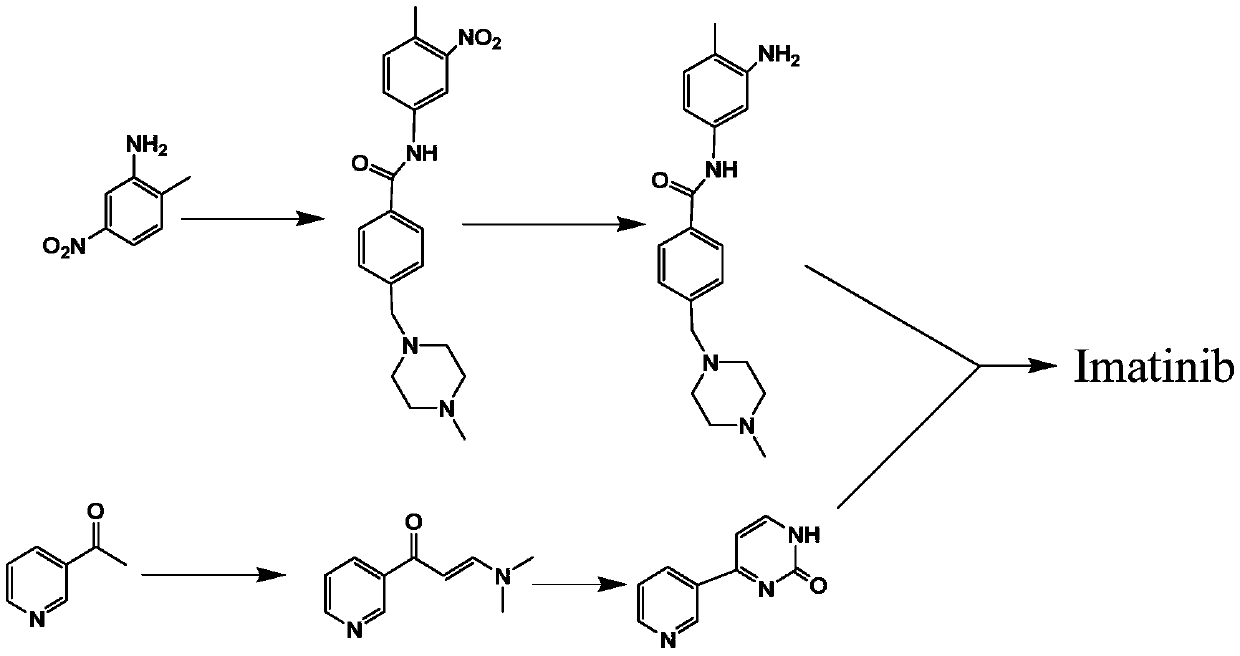
Synthesis method of imatinib and imatinib mesylate
A technology of methanesulfonate and synthesis method, applied in the directions of sulfonate preparation, organic chemistry, etc., can solve the problems of long production cycle, increased cost, complicated and complicated post-processing, etc., and achieve the effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Synthesis of 3-dimethylamino-1-(3-pyridyl)-2-propen-1-one
[0066] Add 96.91g of 3-acetylpyridine and 104.86g of N,N-dimethylamide dimethyl acetal into a 500ml round-bottomed flask, raise the temperature to 85°C and react for 8h. After the reaction, unreacted N , N-dimethylamide dimethyl acetal, cooled and crystallized, filtered with suction, washed the filter cake with ether, and dried naturally to obtain 132.89 g of a yellow-brown solid with a yield of 94.38%.
[0067] Mp: 81~82℃
[0068] 1 H-NMR (CDCl 3 )δ: 9.05 (1H, d, J = 1.6Hz), 8.68 (1H, dd, J = 4.8, 1.6 Hz), 8.21 (1H, m), 7.86 (1H, d, J = 12.2Hz,), 7.37 (1H,dd,J=7.3, 4.8Hz), 5.69(1H,d,J=12.2Hz), 3.20(3H,s), 2.97(3H,s).
[0069] MS:177.3(M+H)
[0070] Purity: TLC (ethyl acetate: petroleum ether = 1:1) single point
[0071] Add 1.45kg of 3-acetylpyridine and 1.57kg of N,N-dimethylamide dimethyl acetal to a 10L reaction kettle respectively, raise the temperature to 85°C and react for 10h. After the re...
Embodiment 2
[0072] Example 2 Synthesis of 2-methyl-5-nitrophenylguanidine nitrate
[0073] Throw 121.72g of 2-methyl-5-nitroaniline into the medium, then throw 100.8g of 50% NH 2 CN, add 160ml of ethanol, stir and heat up to 70°C, then add 118ml of concentrated hydrochloric acid dropwise into the three-necked flask, reflux for 3 hours after the dropwise addition, after the reaction, cool down to 45°C, pour 50ml of concentrated HNO 3 Continue to stir for about 1 min, let stand to cool down and crystallize, filter with suction, wash the filter cake with 150 ml of ethanol, and dry to obtain 179.16 g of yellow-white solid with a yield of 87.07%.
[0074] Mp: 210~211℃
[0075] 1 H-NMR (DMSO-d 6 )δ: 8.63-8.64 (1H, br), 7.21-7.27 (1H, m), 6.72-6.76 (1H, d, J = 1.6Hz), 6.69 (3H, s), 1.43 (3H, s)
[0076] MS:195.2(M+H)
[0077] Throw 2.43kg of 2-methyl-5-nitroaniline into a 20L reactor, and then throw 2.12kg of 50% NH 2 CN, add 2.5L ethanol, stir and heat up to 70°C, then add 2.3L concentrat...
Embodiment 3
[0078] Example 3 Synthesis of N-(2-methyl-5-nitrophenyl)-4-(3-pyridyl)-2-pyrimidinamine
[0079] Throw 52.8g of 3-dimethylamino-1-(3-pyridyl)-2-propen-1-one into a three-necked flask, then throw 300ml of ethanol and stir until it is completely dissolved, then add 2-methyl-5 -Nitrophenylguanidine nitrate 77.16g, add 19.44g KOH, react under reflux for 18h, cool to room temperature after the reaction, filter with suction, and wash the filter cake with 50ml of isopropanol, then wash with water until the filtrate is neutral and colorless, dry 80.01 g of yellow-white solid was obtained, and the yield was 86.87%.
[0080] Mp: 193~194℃
[0081] 1 H-NMR (CDCl 3 )δ: 9.48(1H, s), 9.27(1H, s), 8.76-8.74(1H, m), 8.54-8.60(2H, m), 7.85-7.88(1H, m), 7.49-7.52(1H, m), 7.36-7.32 (2H, m), 7.18 (1H, s) 2.48 (3H, s).
[0082] MS:308.3(M+H)
[0083] Purity: TLC (ethyl acetate: acetone = 4:1) single point
[0084] Throw 1.06kg of 3-dimethylamino-1-(3-pyridyl)-2-propen-1-one into the kettle, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com