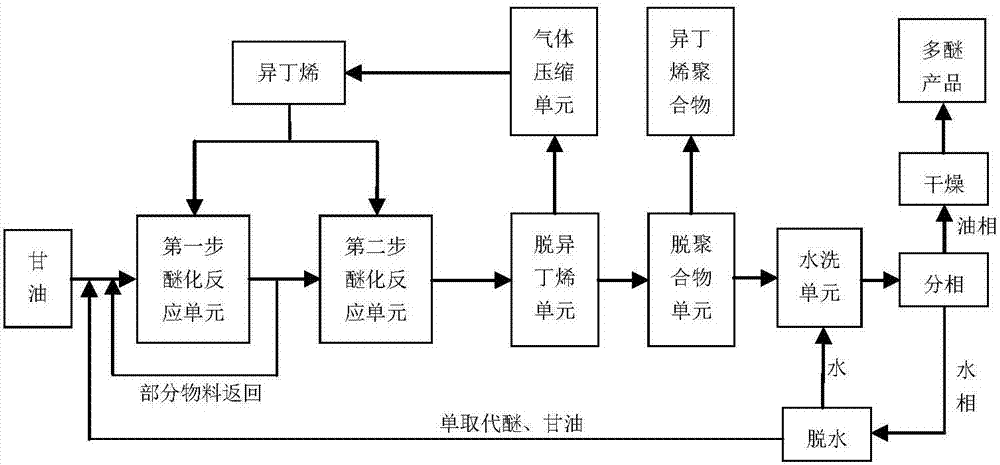
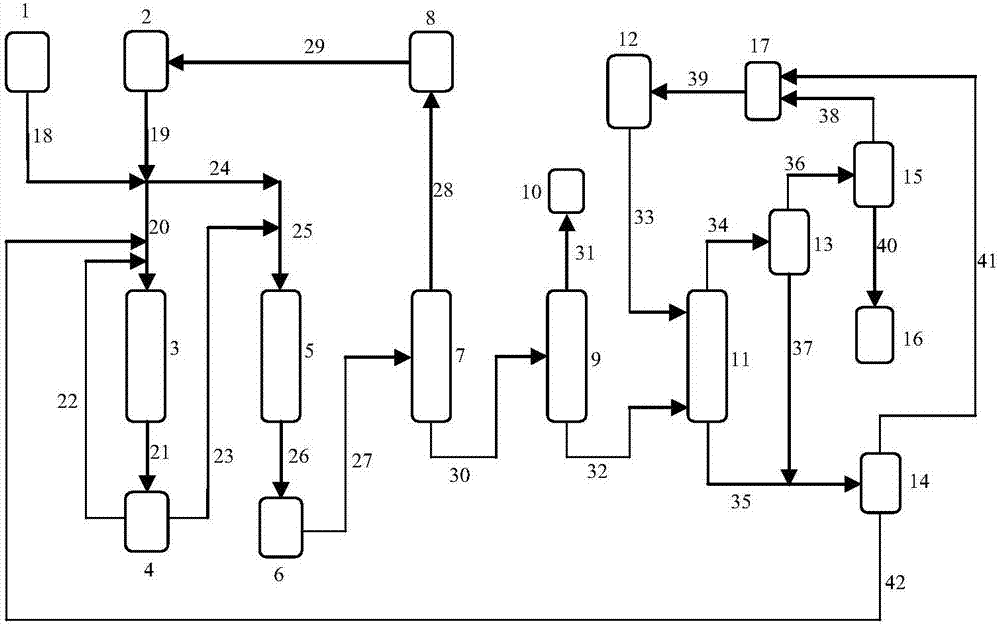
Two-step method and system for preparation of glycerol alkyl ether
A technology of glycerin alkyl ether and glycerin, which is applied in the field of two-step preparation of glycerin alkyl ether, and can solve the problems of long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This example illustrates that the present invention can prepare glycerol tert-butyl ether with isobutylene and glycerol in a two-step process.
[0048] (1) The first step reaction: 10.0 g of glycerin, 0.50 g of macroporous sulfonic acid catalyst KC111 agent (Hebei Kairui Company) and 12.5 g of isobutylene (analytical pure, content 99.5%) were added to a 100 mL reactor, and olefin oil The molar ratio is 2:1. The reaction temperature is 70° C., the reaction pressure is 1.3 MPa, and the reaction is performed for 4.0 hours with electric stirring.
[0049] Gas chromatography analysis shows that the conversion rate of glycerol is 82.9%, the selectivity of monosubstituted tert-butyl glyceryl ether is 23.3%, the selectivity of disubstituted tert-butyl glyceryl ether is 71.4%, the selectivity of trisubstituted tert-butyl glyceryl ether The specificity is 5.3%, and the selectivity of isobutylene dimer is 1.4%.
[0050] (2) The second step reaction: Based on the first step react...
Embodiment 2
[0072] This example illustrates that the present invention can prepare glycerol tert-butyl ether with isobutylene and glycerol in a two-step process.
[0073] (1) The first step reaction: 10.0 g of glycerin, 0.50 g of macroporous sulfonic acid catalyst KC111 agent (Hebei Kairui Company) and 10.0 g of isobutylene (analytical purity, content 99.5%) were added to a 100 mL reactor, and olefin oil The molar ratio is 1.6:1. The reaction temperature is 75° C., the reaction pressure is 1.3 MPa, and the reaction is performed for 4.0 hours with electric stirring.
[0074] Gas chromatography analysis shows that the conversion rate of glycerol is 76.6%, the selectivity of monosubstituted tert-butyl glyceryl ether is 18.5%, the selectivity of disubstituted tert-butyl glyceryl ether is 78.0%, and the selectivity of trisubstituted tert-butyl glyceryl ether is 78.0%. The specificity is 3.5%, and the selectivity of isobutylene dimer is 2.6%.
[0075] (2) The second step reaction: on the basi...
Embodiment 3
[0079] This example illustrates that the present invention can use a two-step method to prepare glycerol tert-butyl ether using a fixed-bed reactor.
[0080] 10.0 g of macroporous sulfonic acid catalyst KC111 agent (Hebei Kairui Chemical Co., Ltd.) was filled in the fixed-bed reactor on the high-pressure micro-reactor test device (catalyst bed height 22 cm).
[0081] (1) The first step reaction: glycerol feed rate 6.7g / h, isobutene feed rate 8.3g / h (n isobutene:n glycerol=2:1), liquid hourly space velocity is 0.67h -1 , the residence time is 1.5h, the temperature of the fixed bed reactor is 70°C, and the nitrogen back pressure is 2.0MPa.
[0082] Gas chromatography analysis shows that the conversion rate of glycerol is 85.0%, the selectivity of monosubstituted tert-butyl glyceryl ether is 12.8%, the selectivity of disubstituted tert-butyl glyceryl ether is 70.4%, the selectivity of trisubstituted tert-butyl glyceryl ether The specificity is 16.8%, and the selectivity of isobu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com