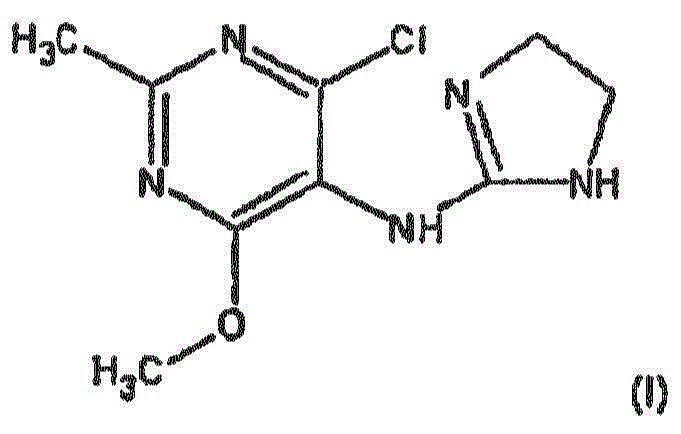
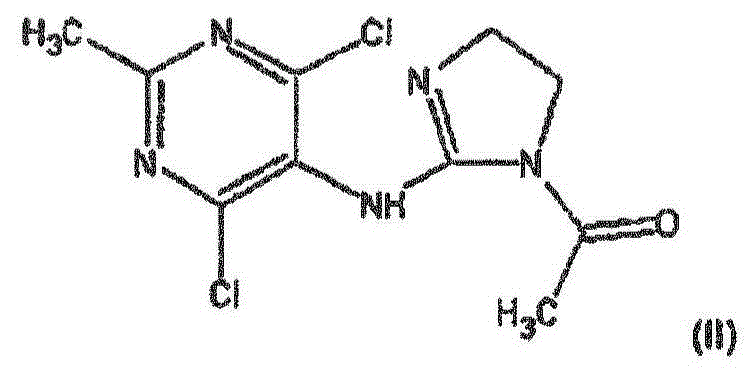
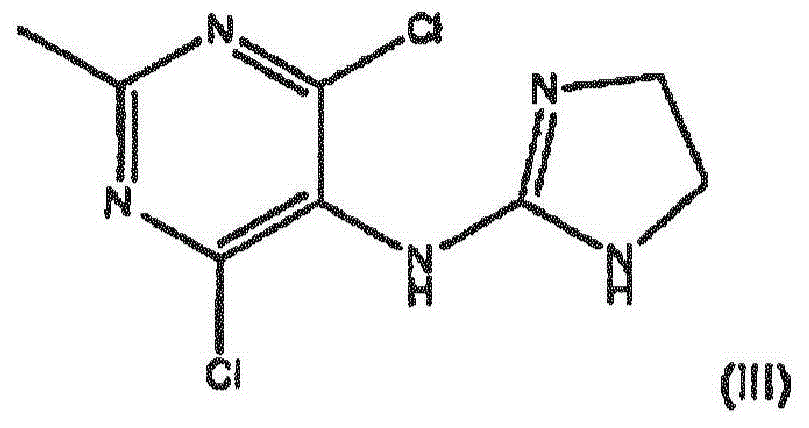
Improved process for the manufacture of moxonidine
A mixture and technology within minutes, applied in the direction of organic chemistry, etc., can solve the problems of no public synthesis method of moxonidine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example
[0053] Preparation Examples 1 to 3 Preparation of Moxonidine
[0054] 102 kg (128 L, 3014.4 mol) of methanol and 7.7 kg (55.6 mol) of potassium carbonate were injected into the reactor, and the mixture was vigorously stirred and heated to 65°C. 8kg DMAIA (27.8mol) was divided into 8 portions and added within 20 minutes. The contents of the reactor were kept at 65°C with stirring for an additional 7 to 12 minutes. The reaction was stopped by transferring the entire reaction mixture to a separate pre-cooled reactor in less than 1 minute and further cooling the reaction mixture to below 25°C in 20 minutes.
[0055] work process:
[0056] The pH value is adjusted to pH≤7 with acetic acid. The reaction mixture is optionally filtered through activated carbon. Then the pH of the filtrate was adjusted to pH ≥ 8 with aqueous ammonia solution, and moxonidine was separated, washed with water and methanol, and dried.
[0057] Yield: Theoretically 92.0%
[0058] Table 1: Moxonidine impuritie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com