8-cyclohexyl-2-fluoro-vidarabine as well as preparation method and application thereof
A technology of arabinoside nucleoside derivatives and compounds, which is applied in the fields of chemistry and medicine, can solve problems such as long history, poor treatment effect, and large drug resistance, and achieve the effect of simplifying synthesis operations and avoiding heavy metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
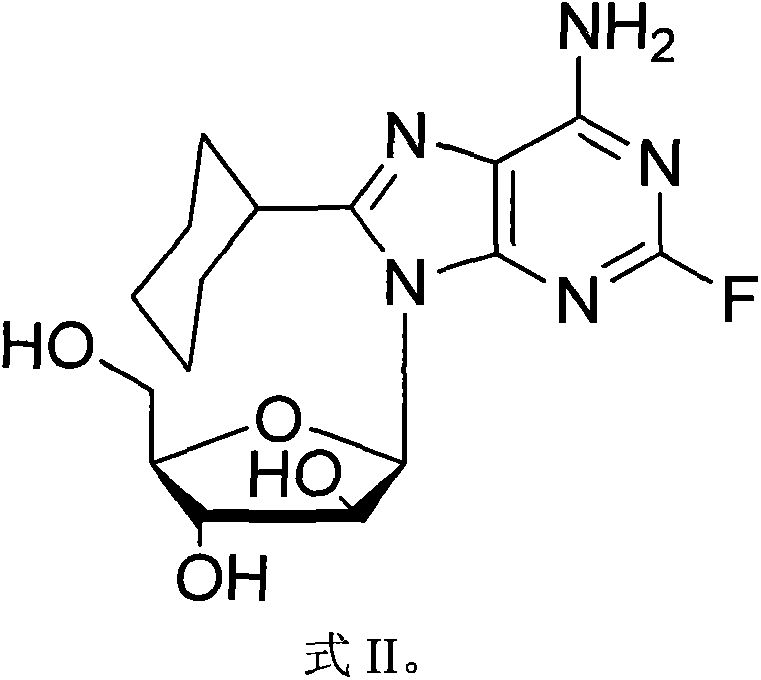
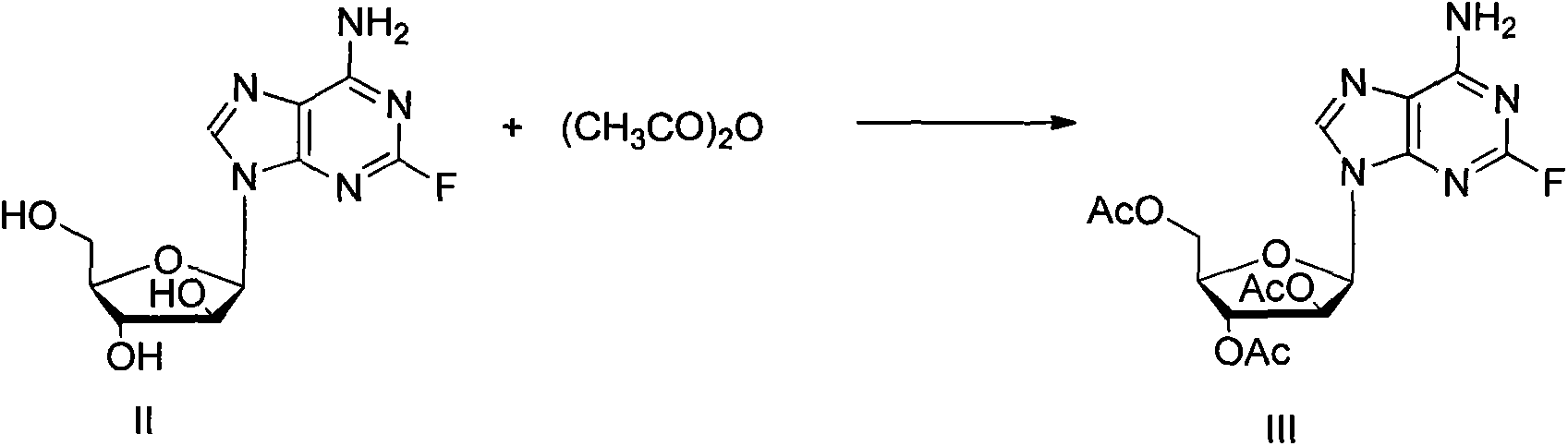
[0021] In a 50mL round bottom flask, add 2-fluoro vidarabine (II, 0.29g, 1mmol), triethylamine (6.3mL, 4.5mol), 4-diaminopicoline (0.02g, 0.16mmol) and 20mL of acetonitrile, ice-water bath, keep the temperature below 5°C, add acetic anhydride (3.4mL, 3.6mmol) dropwise within 10 minutes, remove the ice-water bath, raise the temperature to 60°C, continue the reaction for 1 hour, cool down to room temperature, track with TLC The progress of the reaction shows that the reaction of the raw materials is complete. Slowly add 1 mL of methanol dropwise, remove the solvent on a rotary thin-film evaporator, add 5 mL of ethanol, stir at room temperature for 1 hour, white needle-like crystals precipitate, filter, and wash the filter cake with 5 mL of ethanol. The filter cake was dried to obtain 0.38g, yield 92% (yield is based on II), which is compound III whose hydroxyl group is protected by acetyl group, which is a colorless oil. 1 H NMR (CDCl 3 , 400MHz) δ8.90(s, 1H), 8.27(s, 1H), 6.9...
Embodiment 2
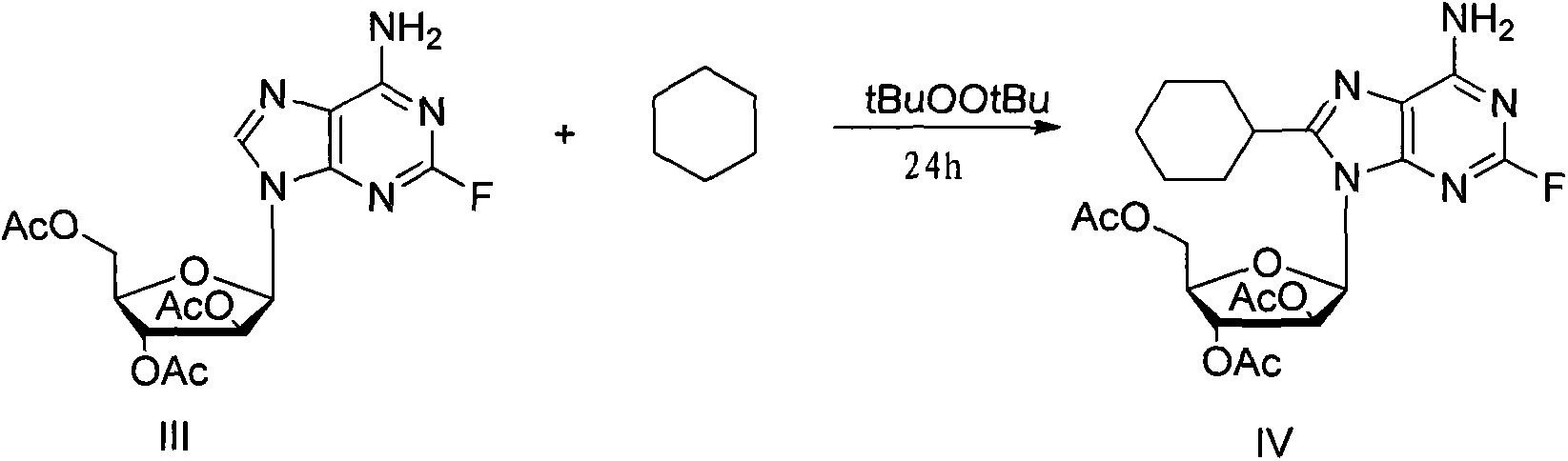
[0023] Hydroxyl-protected compound III (0.21g, 0.5mmol), peroxidized tert-butyl (0.19mL, 1mmol) and 10mL cyclohexane were added to a reaction tube equipped with a Teflon sealing plug, and reacted at 140°C for 24 hours, After cooling to room temperature, the reaction progress was followed by TLC, which showed that the reaction of the raw materials was complete. The solvent was removed on a rotary thin film evaporator, and purified by column chromatography to obtain 0.21 g of oily product 8-cyclohexyl-2-fluoro-triacetyl adenosine IV, with a yield of 85% (yield based on III). The product is a colorless oil. 1 H NMR (CDCl 3 , 400MHz) δ6.23(t, J=4.8Hz, 1H), 5.90-5.85(m, 1H), 5.81(brs, 2H), 4.50-4.47(m, 1H), 4.39-4.33(m, 2H) , 2.82-2.75(m, 1H), 2.15(s, 3H), 2.09(s, 6H), 2.04-1.97(m, 3H), 1.80-1.65(m, 8H). 13 C NMR (CDCl3, 100MHz) δ159.2 (d, J F-C2 =208.4Hz), 156.8, 156.5(d, J F-C4 =11.4Hz), 151.7(d, J F-C8 =19.3Hz), 116.7(d, J F-C5 =3.9Hz), 86.3, 80.0, 72.0, 70.2, 62.6, 36.3,...
Embodiment 3
[0025] Add 8-cyclohexyl-2-fluoro-triacetyl vidarabine IV (0.123g, 0.25mmol) and 10mL ammonia in methanol solution into the reaction bottle, seal it, and react at room temperature for 24 hours. TLC plates followed the progress of the reaction, showing complete reaction of starting materials. Remove the solvent on a rotary thin film evaporator, add methanol for recrystallization, and filter to obtain a white solid, which is 8-cyclohexyl-2-fluoro-adenosine I, 0.16 g, with a yield of 87%. (Yields are based on IV). The resulting product is a white solid. m.p.205-207 ° C. 1H NMR (DMSO-d 6 , 400MHz) δ6.33(t, J=4.8Hz, 1H), 5.91-5.88(m, 1H), 5.89(brs, 2H), 4.51-4.41(m, 1H), 4.36-4.32(m, 2H) , 2.80-2.71(m, 1H), 2.14-1.99(m, 3H), 1.82-1.66(m, 8H); 13 CNMR (DMSO-d 6 , 100MHz) δ159.8 (d, J F-C2 =208.0Hz), 157.0, 156.9(d, J F-C4 =11.4Hz), 152.0(d, J F-C8 =19.3Hz), 115.8(d, J F-C5 =3.9Hz), 86.5, 80.9, 71.7, 70.3, 62.5, 36.1, 31.0, 29.6, 25.5; HRMS: calacd for C 16 h 23 FN 5 o 4 [...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com