Preparation method of fosphenytoin sodium intermediate
A technology of fosphenytoin sodium and intermediates, applied in the field of pharmaceutical synthesis, can solve the problems of low process yield, unsatisfactory mass production, increased production cost and the like, and achieve the effects of simplified operation steps, high production efficiency and economical reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
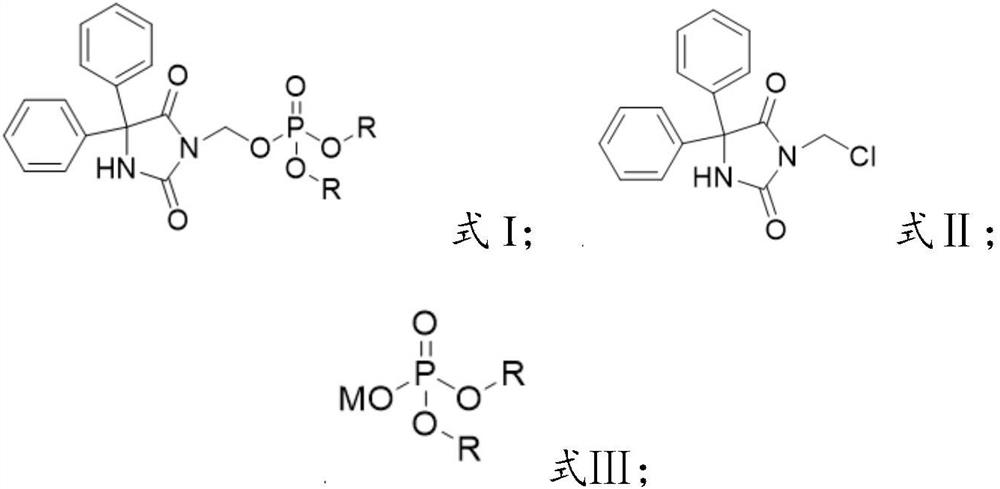
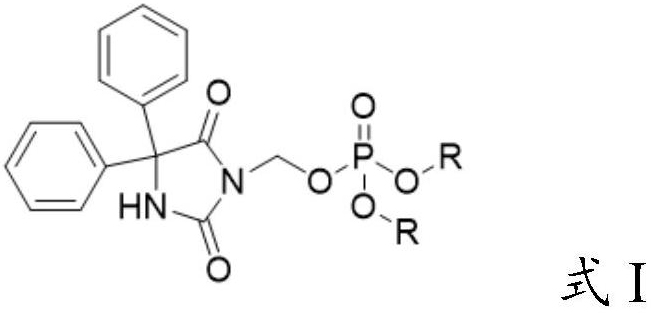
[0036] The invention provides a preparation method of a fosphenytoin sodium intermediate, comprising the following steps:
[0037] A) under the action of a catalyst, react the compound represented by formula II with the compound represented by formula III in solvent a;
[0038] B) mixing the reacted product of step A) with solvent b, cooling down, and crystallizing to obtain the compound represented by formula I;
[0039]
[0040] Wherein, R is selected from C1~C6 alkyl or aralkyl;
[0041] M is an alkali metal ion.
[0042] In certain embodiments of the present invention, the R is selected from methyl, ethyl, tert-butyl or benzyl.
[0043] In certain embodiments of the present invention, the M is Na ion or K ion.
[0044] The present invention has no special limitation on the source of the compound represented by the formula II (3-chloromethylphenytoin). prepared by the method in .
[0045] In some embodiments of the present invention, the compound represented by the ...
Embodiment 1
[0073] Preparation of compound 3-hydroxymethyl-phenytoin di-tert-butyl phosphate shown in formula I:
[0074] 50.00 g (166.26 mmol) of the compound represented by formula II, 53.67 g (216.14 mmol) of di-tert-butyl phosphate potassium salt and 0.28 g (1.66 mmol) of potassium iodide were added to 300 mL of acetone, and the temperature was raised to 50 °C with stirring for 8 h. Heating was stopped, 350 mL of water was added, the temperature was lowered to -5°C with stirring, and crystallization was maintained for 2 h. After filtration, the filter cake was dried under reduced pressure to obtain 70.00 g of 3-hydroxymethyl-phenytoin di-tert-butyl phosphate with a yield of 88.7%. The HPLC purity was 99.10% and the content was 99.6% (indicating that the inorganic salts can be effectively removed).
Embodiment 2
[0076] Preparation of compound 3-hydroxymethyl-phenytoin di-tert-butyl phosphate shown in formula I:
[0077] 50.00 g (166.26 mmol) of the compound represented by formula II, 49.54 g (199.51 mmol) of di-tert-butyl phosphate potassium salt and 0.18 g (0.50 mmol) of tetrabutylammonium iodide were added to 200 mL of N,N-dimethylformamide , the temperature was raised to 60 °C with stirring, and the reaction was maintained for 3 h. Heating was stopped, 500 mL of water was added, the temperature was lowered to 15°C with stirring, and crystallization was maintained for 2 h. After filtration, the filter cake was dried under reduced pressure to obtain 70.60 g of 3-hydroxymethyl-phenytoin di-tert-butyl phosphate with a yield of 89.5%. The HPLC purity was 99.14%, and the content was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com