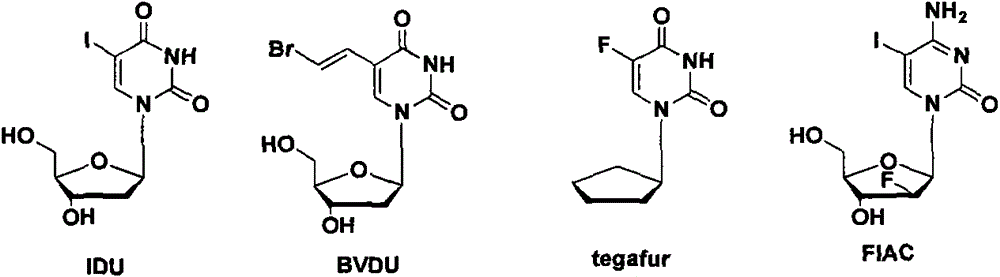
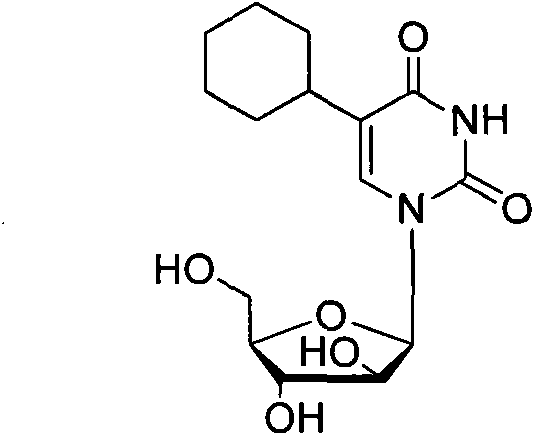
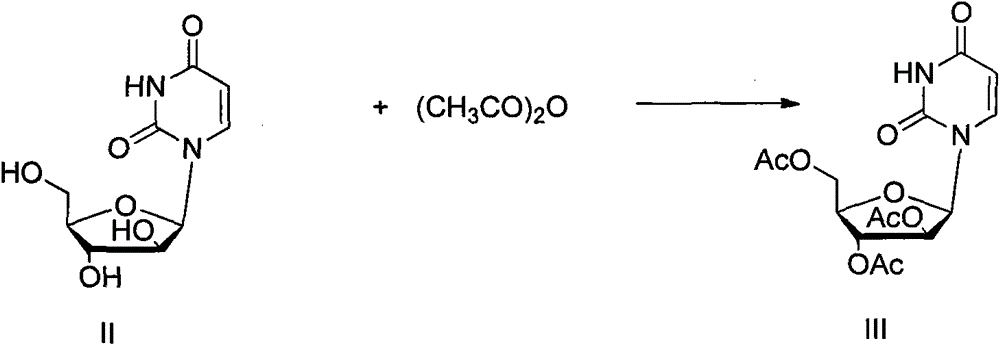5-cyclohexyl uracil arabinoside, preparation method and application thereof
A technology of triacetyl arabinoside and cyclohexyl, applied in the field of its preparation, 5-cyclohexyl arabinoside, can solve the problems of limited anti-leukemia activity and difficult clinical application of arabinoside, to avoid heavy metal residues, The effect of simplifying compositing operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 50mL round bottom flask, add arabouridine (II, 0.244g, 1mmol), triethylamine (6.3mL, 4.5mol), 4-diaminopicoline (0.02g, 0.16mmol) and 20mL of acetonitrile, ice In a water bath, keep the temperature below 5°C, add acetic anhydride (3.4mL, 3.6mmol) dropwise within 10 minutes, remove the ice-water bath, raise the temperature to 60°C, continue the reaction for 1 hour, and cool down to room temperature. The raw material reacted completely. Slowly add 1 mL of methanol dropwise, remove the solvent on a rotary thin-film evaporator, add 5 mL of ethanol, stir at room temperature for 1 hour, white needle-like crystals precipitate, filter, and wash the filter cake with 5 mL of ethanol. The filter cake was dried to obtain 0.35 g with a yield of 95% (yield is based on II), which is the compound whose hydroxyl group is protected by acetyl group.
[0025] Colorless oil. 1 H NMR (CDCl 3 , 400MHz) δ10.38(s, 1H), 7.64(d, J=6.4Hz, 1H), 6.29(d, J=6.4Hz, 1H), 6.17(d, J=5.2Hz, 1H), 5....
Embodiment 2
[0027] Hydroxyl-protected compound (III, 0.14g, 0.5mmol), peroxide and tert-butyl (0.19mL, 1mmol) and 10mL cyclohexane were added to a reaction tube equipped with a Teflon sealing plug, and reacted at 140°C for 24 hours , cooled to room temperature, TLC traced the reaction process, showing that the reaction of the raw materials was complete. The solvent was removed on a rotary thin-film evaporator, and purified by column chromatography to obtain 0.19 g of oily product 5-cyclohexyl-triacetylararabine, with a yield of 87% (yield based on III), namely compound IV.
[0028] Colorless oil. 1 H NMR (CDCl 3 , 400MHz) δ9.60(s, 1H), 7.16(s, 1H), 6.29(d, J=4.0Hz, 1H), 5.37-5.36(m, 1H), 5.09(d, J=2Hz, 1H) , 4.41(d, J=5.2Hz, 1H), 4.17-4.14(m, 1H), 2.59-2.53(m, 1H), 2.12(s, 3H), 2.09(s, 3H), 2.06(s, 3H ), 1.85-1.70(m, 6H), 1.22-1.01(m, 4H); 13 C NMR (CDCl 3 , 100MHz) δ170.5, 169.7, 168.4, 163.1, 149.8, 135.0, 119.5, 84.1, 80.3, 76.4, 74.7, 62.6, 34.8, 32.4, 26.5, 26.4, 26.0, 20.7, 20....
Embodiment 3
[0030] Add 5-cyclohexyl-triacetylararabine (IV, 0.22 g, 0.5 mmol) and 10 ml of ammonia in methanol solution into the reaction bottle, seal it, and react at room temperature for 24 hours. TLC plates followed the progress of the reaction, showing complete reaction of starting materials. Remove the solvent on a rotary thin film evaporator, add methanol for recrystallization, and filter to obtain a white solid, which is 0.16 g of 5-cyclohexyl-arabinuridine, with a yield of 82% (the yield is based on IV).
[0031] The product obtained was a white solid.. 1 H NMR (DMSO-d 6 , 400MHz) δ9.67(s, 1H), 7.12(s, 1H), 6.18(d, J=5.2Hz, 1H), 5.35-5.34(m, 1H), 5.14(d, J=2Hz, 1H) , 4.45(d, J=5.2Hz, 1H), 4.17-4.15(m, 1H), 2.60-2.57(m, IH), 1.88-1.72(m, 6H), 1.24-1.08(m, 4H); 13 C NMR (CDCl 3 , 100MHz) δ163.8, 148.7, 135.7, 120.5, 86.1, 80.7, 76.8, 74.0, 62.3, 34.8, 32.5, 26.2, 26.0, 25.8; HRMS calcd for C 15 h 23 N 2 o 6 [M+H + ] 327.1551, found 327.1550.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com