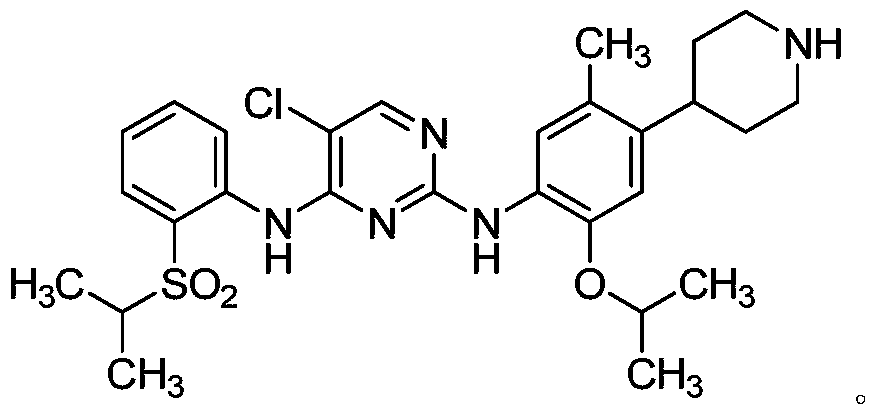
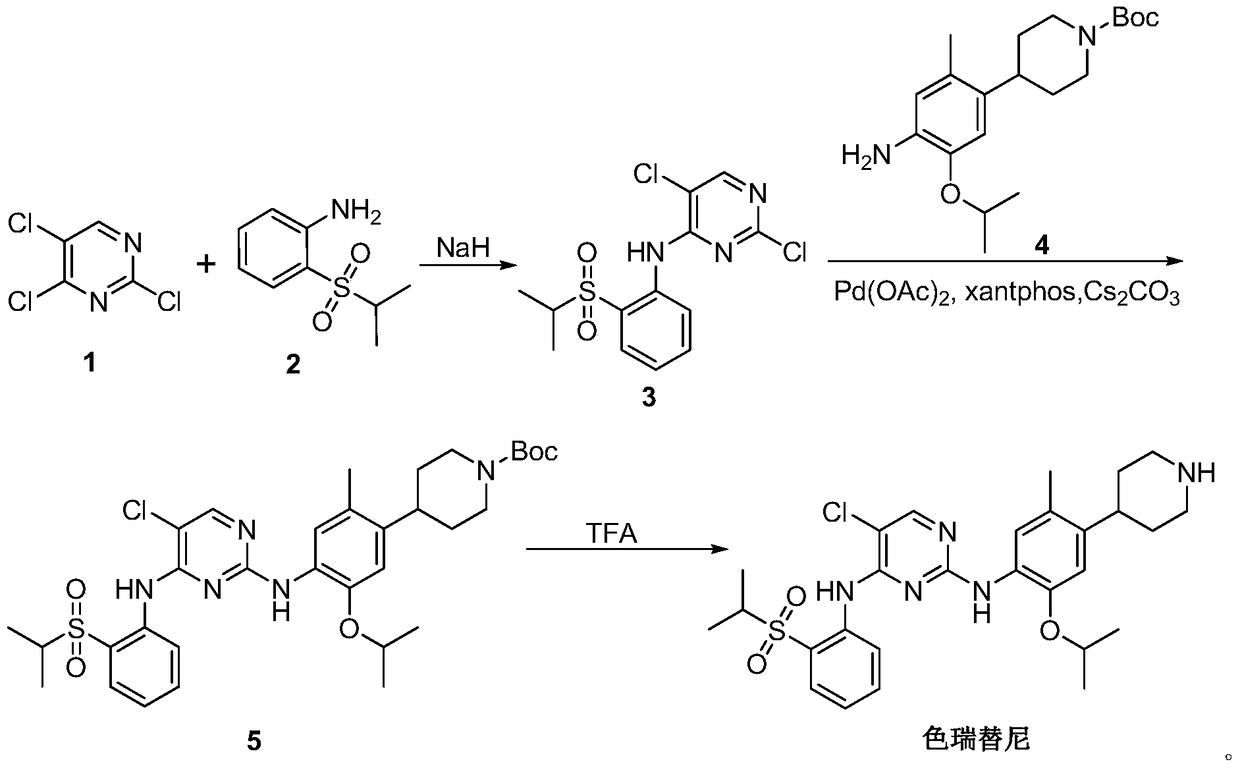
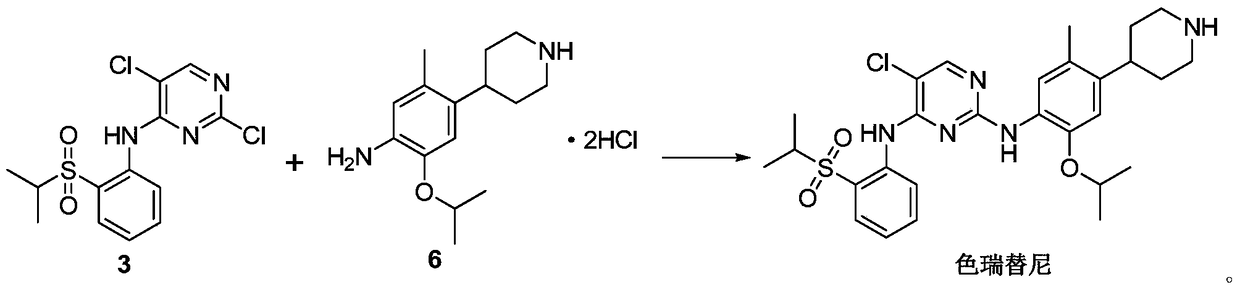
A kind of preparation method of ceritinib and its intermediate
A technology for ceritinib and a compound, which is applied in the field of preparation of ceritinib, can solve the problems of complicated purification operation, high reaction temperature, difficult industrial expansion of production, etc., and achieves improved reaction yield, mild reaction conditions, and avoidance of heavy metals residual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of formula III compound
[0040]
[0041] Dissolve the compound of formula I (117g, 0.603mol) and the compound of formula II (100g, 0.502mol) in 1500mL of chloroform, add diisopropylethylamine (78g, 0.60mol) at room temperature, and heat to reflux for 2 hours . After the reaction was detected by TLC, it was concentrated to dryness to obtain 300 g of a brown solid. Then add 600mL of methanol, stir well at 10°C, and filter with suction to obtain 152g of light yellow compound of formula III, the HPLC purity is greater than 98%, and the yield is 85%. 1 H-NMR (300MHz, CDCl 3 )δ:11.56(s,1H),9.26(s,1H),8.26(d,1H),8.03(d,1H),7.76(m,1H),7.48(m,1H),3.21(m,1H ),1.33(d,6H).MS:379.0[M+Na] + ,355.0[M-H] - .
Embodiment 2
[0042] Embodiment 2: the synthesis of formula V-Boc compound
[0043]
[0044] The compound of formula III (60g, 0.168mol) and the compound of formula IV-Boc (64.5g, 0.185mol) were dissolved in 1800mL of absolute ethanol, heated to 60°C for 8 hours, after the reaction was detected by TLC, concentrated to dryness to obtain a brown color solid. Recrystallization with PE:EA=3:1 gave 91 g of the compound of formula V-Boc as a yellow solid, with an HPLC purity greater than 98% and a yield of 81%.1 H-NMR (300MHz, CDCl 3 )δ:11.58(s,1H),9.21(s,1H),8.15(m,3H),7.87(d,1H),7.64(d,1H),7.46(m,1H),6.73(m,1H ),4.28(m,1H),4.14(m,2H),3.28(m,1H),2.82(m,3H),1.93(m,2H),1.72(m,2H),1.51(m,3H) ,1.39(m,10H),1.28(m,11H).MS:669.3[M+H] + ,691.3[M+Na] + .
Embodiment 3
[0045] Embodiment 3: the synthesis of formula VI-Boc compound
[0046]
[0047] A mixture of formula V-Boc compound (20g, 0.030mol), reduced iron powder (8.1g, 0.145mol), ammonium chloride (15.5g, 0.290mol) dissolved in 600mL ethanol and water (V:V=2:1) The solution was heated to reflux for 1 h. After the reaction was detected by TLC, it was filtered, and the filtrate was concentrated to dryness to obtain 16.5 g of a gray-green solid. The HPLC purity was greater than 98%, and the yield was 86%. 1 H-NMR (300MHz, CDCl 3 )δ:9.49(s,1H),8.70(d,1H),8.13(s,1H),7.91(m,2H),7.64(d,1H),7.41(m,1H),7.28(m,1H ),6.72(m,1H),4.56(m,1H),4.27(m,2H),3.30(m,1H),2.84(m,3H),2.23(m,3H),1.93(m,2H) ,1.75(m,2H),1.52(m,2H),1.39(m,10H),1.34(m,11H).MS:639.3[M+H] + ,661.3[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com