Preparation method of timolol impurity
A timolol and impurity technology, applied in the field of timolol impurity preparation, can solve the problems such as the public report of no impurity synthesis route, and achieve the effect of simple operation, high purity and quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
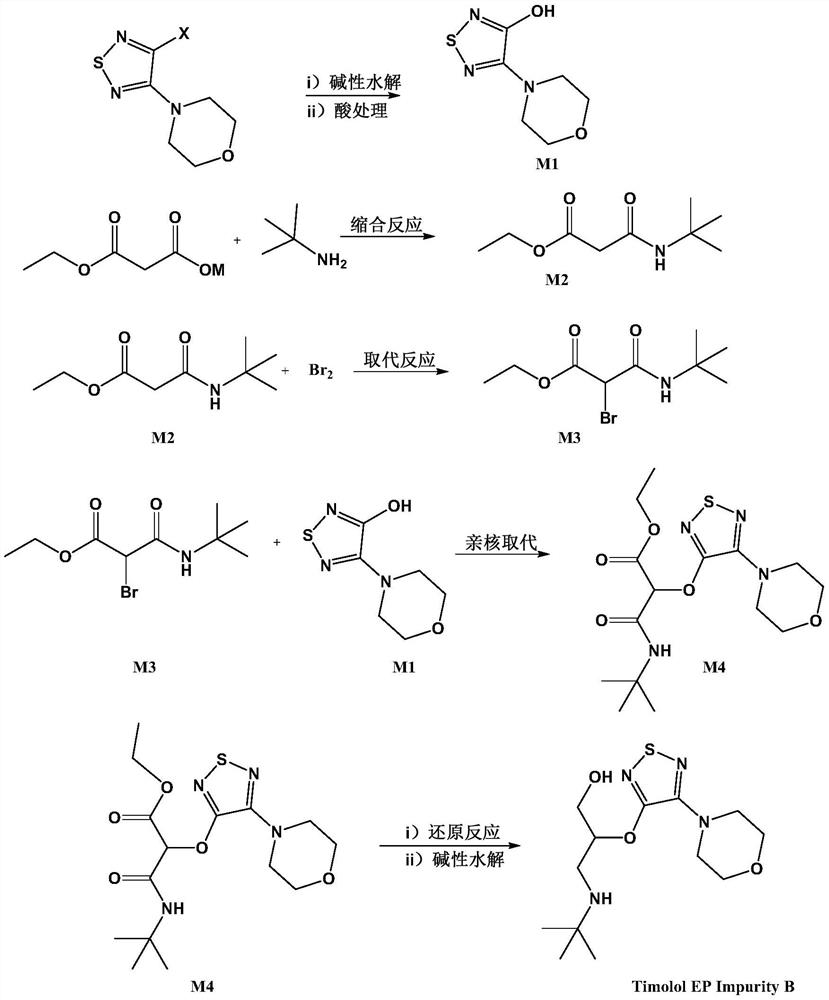
[0031] The present embodiment 1 provides a kind of preparation method of timolol impurity, specifically comprises the following process:
[0032] step 1):
[0033]
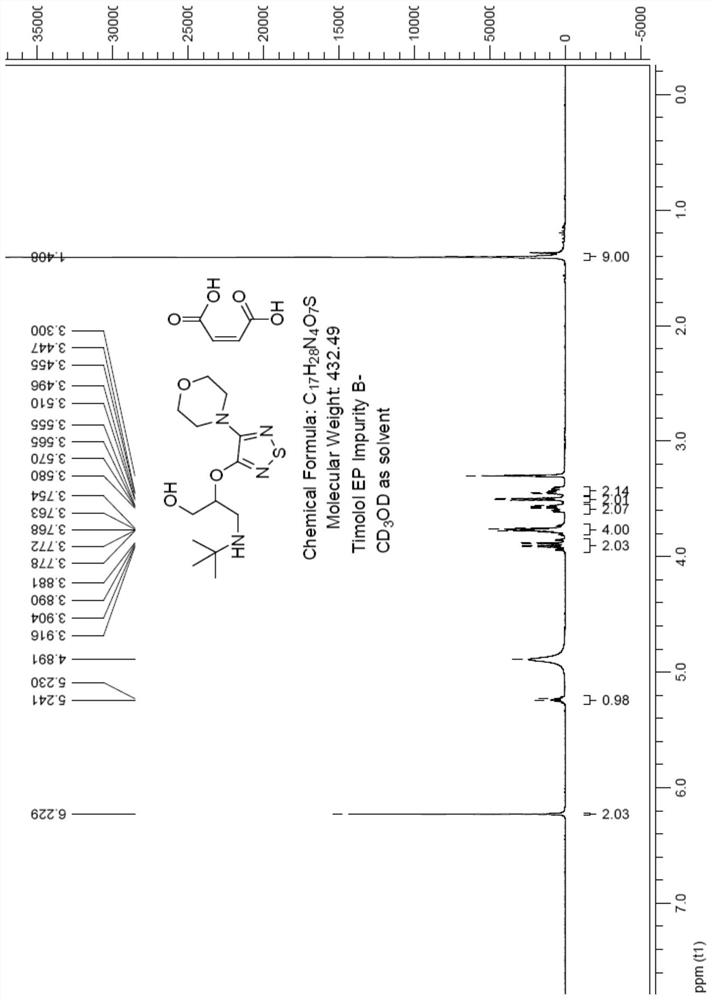
[0034] Add 50 g of 3-chloro-4-morpholino-1,2,5-thiadiazole to 200 ml of dimethyl sulfoxide, add 500 ml of aqueous solution containing 80 g of sodium hydroxide, and react at 120°C for 5 hours. Cool the reaction solution to room temperature, adjust the pH to 1 with dilute hydrochloric acid, filter the precipitated solid, and dry the solid in an oven to obtain intermediate M1 (95% yield);
[0035] Step (2):
[0036]
[0037] Monoethyl malonate potassium salt 30g, 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride 40g, tert-butylamine 15g, 4-dimethylaminopyridine 2.5g, dichloro Add 400ml of methane into the reaction flask, stir at room temperature for 12 hours, add 500ml of aqueous hydrochloric acid solution with an equivalent concentration of 1N for washing, dry the organic phase and evaporate to dryne...
Embodiment 2
[0048] This Example 2 provides a preparation method of Apremilast impurity, the difference from Example 1 is only: 3-halo-4-morpholinyl-1,2,5-thiadiazole in step (1) It is 3-bromo-4-morpholino-1,2,5-thiadiazole, the monoethyl malonate metal salt in step (2) is monoethyl malonate sodium salt, and the condensing agent is N,N -Carbonyldiimidazole.
Embodiment 3
[0050] This Example 3 provides a preparation method of Apremilast impurity, the difference from Example 1 is only: 3-halo-4-morpholinyl-1,2,5-thiadiazole in step (1) 3-iodo-4-morpholino-1,2,5-thiadiazole, monoethyl malonate metal salt is monoethyl malonate sodium salt in step (2), and the condensing agent is 2-( 7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com