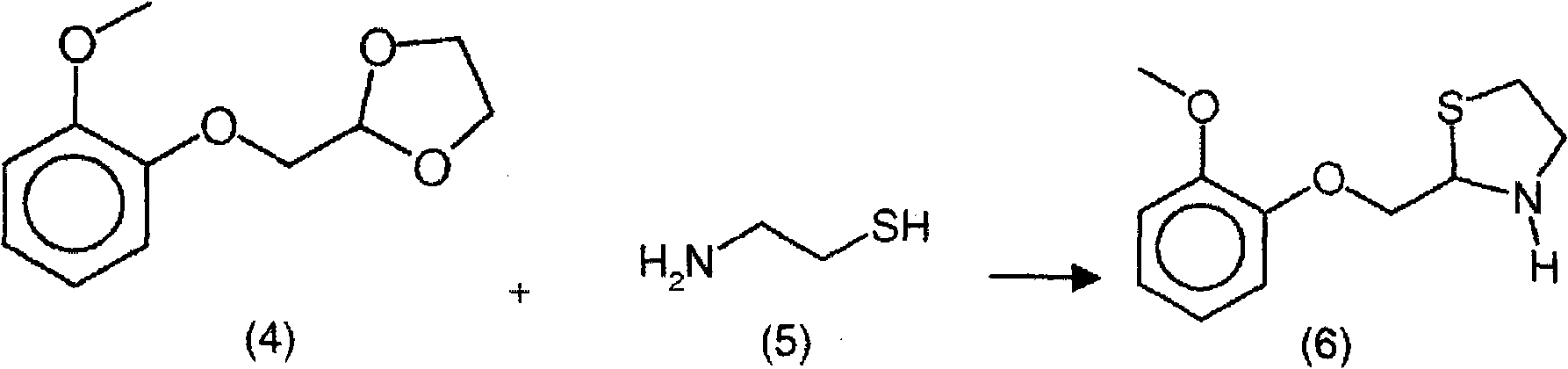
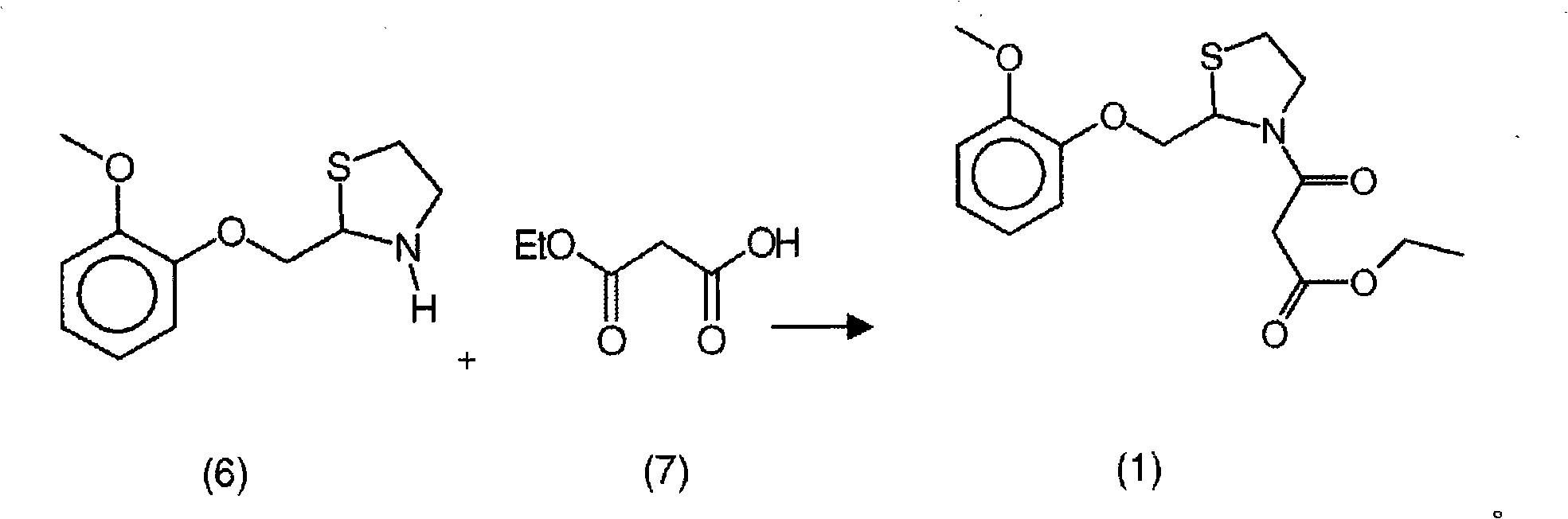
New process for the synthesis of moguisteine
A technology of methyl and methoxyphenoxy, which is applied in the new field of synthesizing mogisteine, which can solve the problems of removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
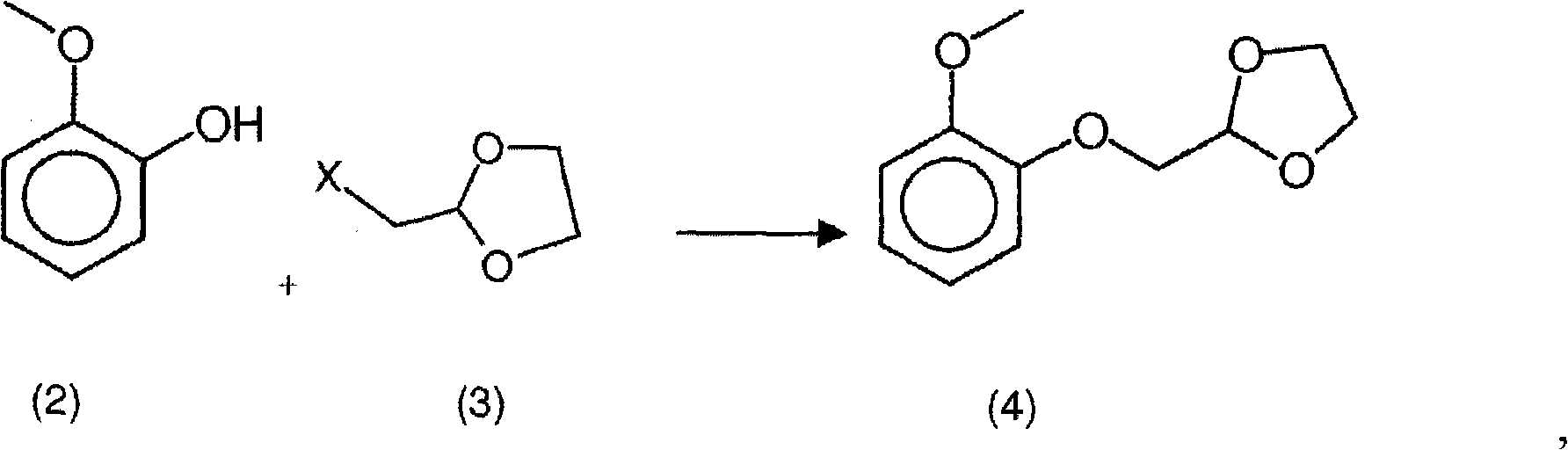
[0070] Step a) Preparation of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane in 1-methoxy-2-propanol Intermediate of ring (4) (4)
[0071] The raw materials were used in the amounts shown in Table 1 below.
[0072] Table 1: Substances and amounts of Example 1
[0073] raw material
density
g
ml
Moore
The molar ratio of
1.129
297.6
263.6
2.40
1.00
1-methoxy-2-propanol
0.920
607.2
660.0
*
*
497.6
3.60
1.50
97% 2-bromomethyl-1,3-dioxide
Pentacyclic
1.628
475.2
291.9
2.76
1.15
50% KOH solution
1.516
84.2
55.5
0.75
0.31
Deionized water, first serving
1.00
660.0
660.0
*
*
Deionized water, second portion
1.00
1320.0
1320.0
*
*
[0074] raw material
density
g
ml
Moore
The molar ratio of
Deionized water for washing
1.00
2x500
2x500
*
*
[0075] Under a nitrogen atmosphere, load potassium carbonate fine powder and 1-methoxy-2-propanol into a completely dry 6-liter flask a...
Embodiment 2
[0101] Step a) Preparation of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane in N-methylpyrrolidone Intermediate of ring (4) (4)
[0102] The following raw materials were used in the amounts shown in Table 2.
[0103] Table 2: Substances and amounts used in Example 2
[0104] raw material
density
g
ml
Moore
The molar ratio of
1.129
99.2
87.9
0.80
1.00
N-methylpyrrolidone
1.028
514.0
500.0
*
*
221.14
1.60
2.00
97% 2-bromomethyl-1,3-dioxide
Pentacyclic
1.628
158.4
97.3
0.92
1.15
Deionized water for quenching
1.00
1400.0
1400.0
Toluene for extraction
0.865
692.0
800.0
Deionized water, first wash
1.00
800.0
800.0
30% NaOH solution
1.335
50.0
37.5
Deionized water, second wash
1.00
800.0
800.0
30% NaOH solution
1.335
50.0
37.5
Deionized water, third wash
1.00
800.0
800.0
30% NaOH solution
1.335
50.0
37.5
carbon
5.0
[0105] raw material
density
g
ml
Moore
Th...
Embodiment 3-31
[0116] Step a) Intermediate (4) of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane (4) preparation
[0117] By following the same procedure as shown in Example 1, but using the amount, reaction solvent, time, and temperature shown in Table 3, intermediate (4) was obtained in the yield and purity shown in Table 4 below.
[0118] Table 3: Reaction conditions of Example 3-31
[0119]
[0120]
[0121]
[0122]
[0123]
[0124] Table 4: Yield and purity of intermediate (4) obtained in Example 3-31
[0125]
[0126]
[0127] Therefore, in all the examples, intermediate (4) was obtained in high yield and with a purity greater than 99%. According to the present invention, the intermediates of Examples 1, 28, 29 and 31 have the best appearance and quality.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com