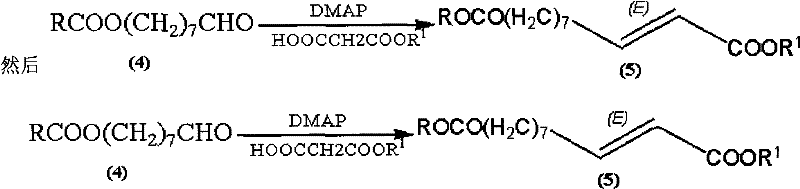
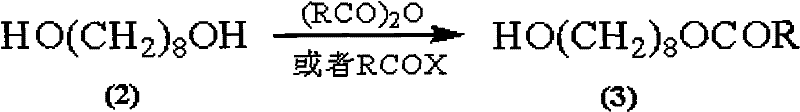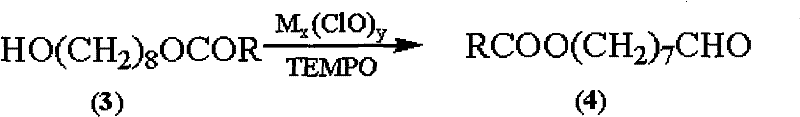Synthetic method of royaljelly acid
A synthesis method and a royal jelly acid technology, applied in the field of medicine, can solve the problems of poor selectivity, difficult product purification, increased side reactions, etc., and achieve the effects of high stereoselectivity, serious environmental pollution, and improved stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 8-acetoxy-1-octanol (3)
[0044] 1,8-octanediol (10.00 g, FW: 146.22; 68.40 mmol) was dissolved in 100 ml of ethyl acetate, and then 100 g of silica (100 mesh) was added to the solution, stirred, and then the solvent was concentrated Removal, to obtain 1,8-octanediol adsorbed on the silica.
[0045] At the same time, in a 1000mL three-necked round bottom flask equipped with mechanical stirring, thermometer and constant pressure dropping funnel, the above adsorbent, 500ml cyclohexane and acetyl chloride (6.44g, FW: 78.50; 82.10mmol) were added to the system in sequence in. The reaction temperature was then raised to 60°C, and stirring was continued at this reaction temperature for 2 hours. After the reaction was completed, the temperature of the system was cooled to room temperature, and then the mixture was filtered, and the filter cake was washed thoroughly with ethyl acetate until the filter cake had no product. A colorless filtrate is obtained, the organ...
Embodiment 2
[0047] Preparation of 8-acetoxy-1-octanol (3)
[0048] 1,8-octanediol (10g, FW: 146.22; 68.40mmol) was dissolved in 100 ml of ether, and then 50 g of silica (100 mesh) was added to the solution, stirred, and then the solvent was concentrated and removed to obtain 1,8-octanediol is adsorbed on silica.
[0049] At the same time, in a 1000 mL three-necked round bottom flask equipped with mechanical stirring, thermometer, and constant pressure dropping funnel, the above-mentioned adsorbent, 500 ml of petroleum ether, and acetyl chloride (10.74g, FW: 78.50; 136.81mmol) were sequentially added to the system. . The reaction was then stirred at 20°C for 12 hours. After the reaction was completed, the temperature of the system was cooled to room temperature, and then the mixture was filtered, and the filter cake was washed thoroughly with ethyl acetate until the filter cake had no product. A colorless filtrate is obtained, the organic phases are combined and the filtrate is distilled und...
Embodiment 3
[0051] Preparation of 8-propionyloxy-1-octanol (3)
[0052] 1,8-octanediol (10g, FW: 146.22; 68.40mmol) was dissolved in 100 ml of ether, and then 100 g of silica (100 mesh) was added to the solution, stirred, and then the solvent was concentrated and removed to obtain 1 , 8-octanediol is adsorbed on silica.
[0053] At the same time, in a 1000 mL three-necked round bottom flask equipped with mechanical stirring, thermometer, and constant pressure dropping funnel, the above adsorbent, 500 ml petroleum ether, and propionyl chloride (12.66g, FW: 92.52; 136.81mmol) were added to the system in sequence in. The reaction was then continued to stir at 60°C for 4 hours. After the reaction was completed, the temperature of the system was cooled to room temperature, and then the mixture was filtered, and the filter cake was washed thoroughly with ethyl acetate until the filter cake had no product. A colorless filtrate is obtained, the organic phases are combined and the filtrate is distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com