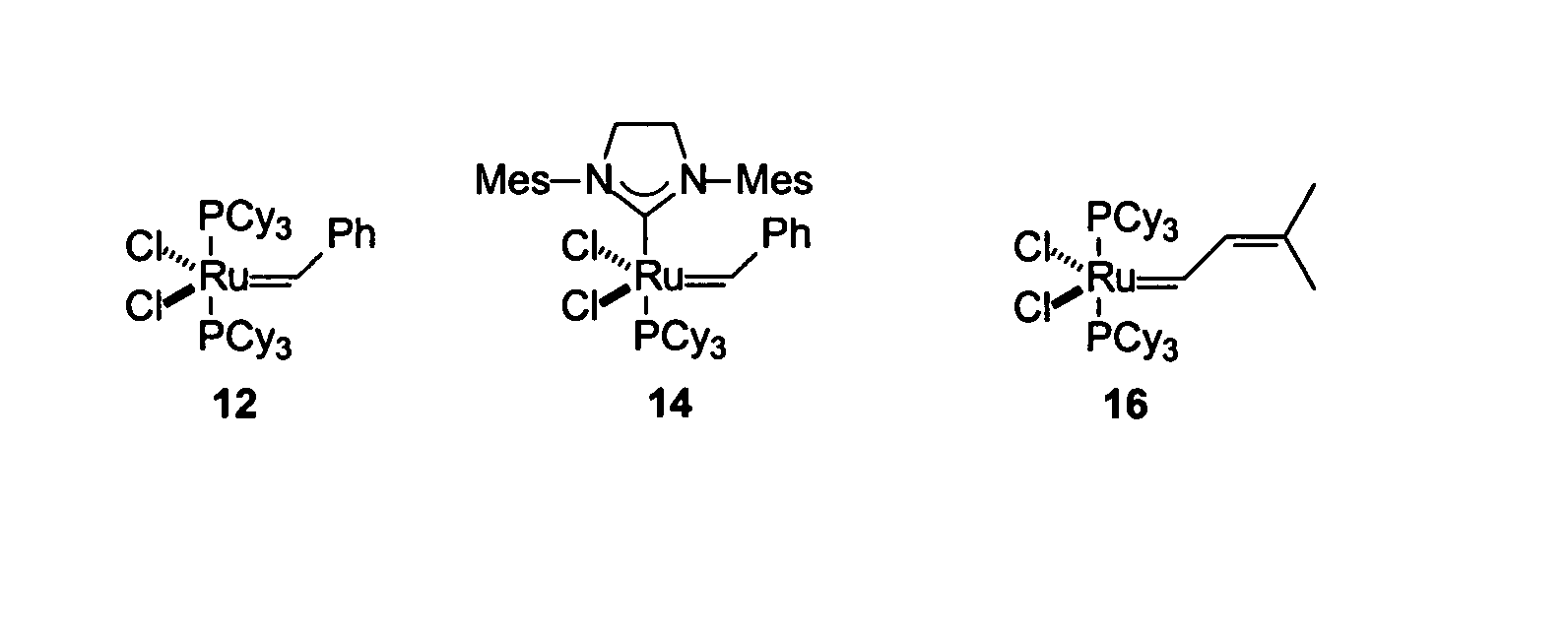
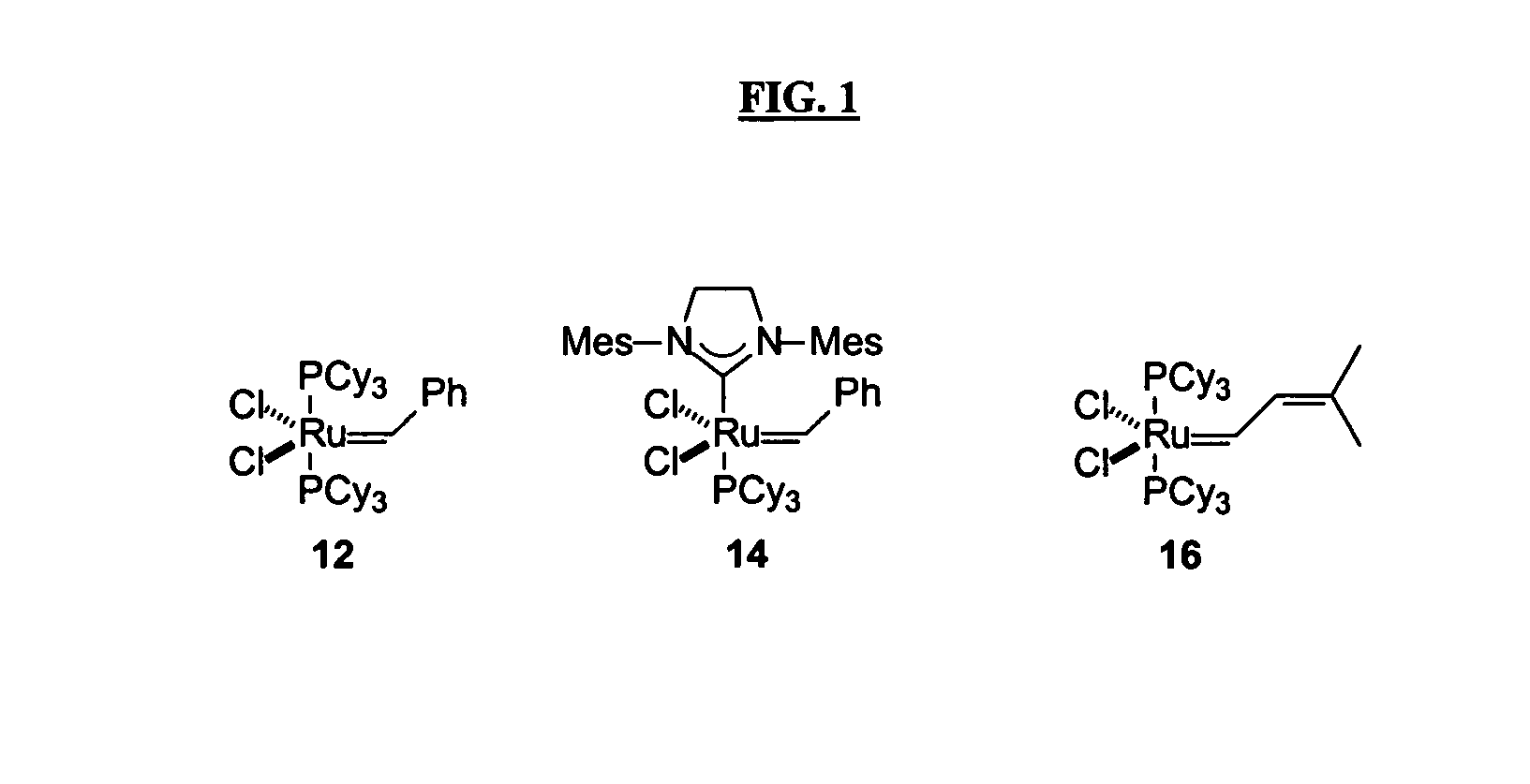
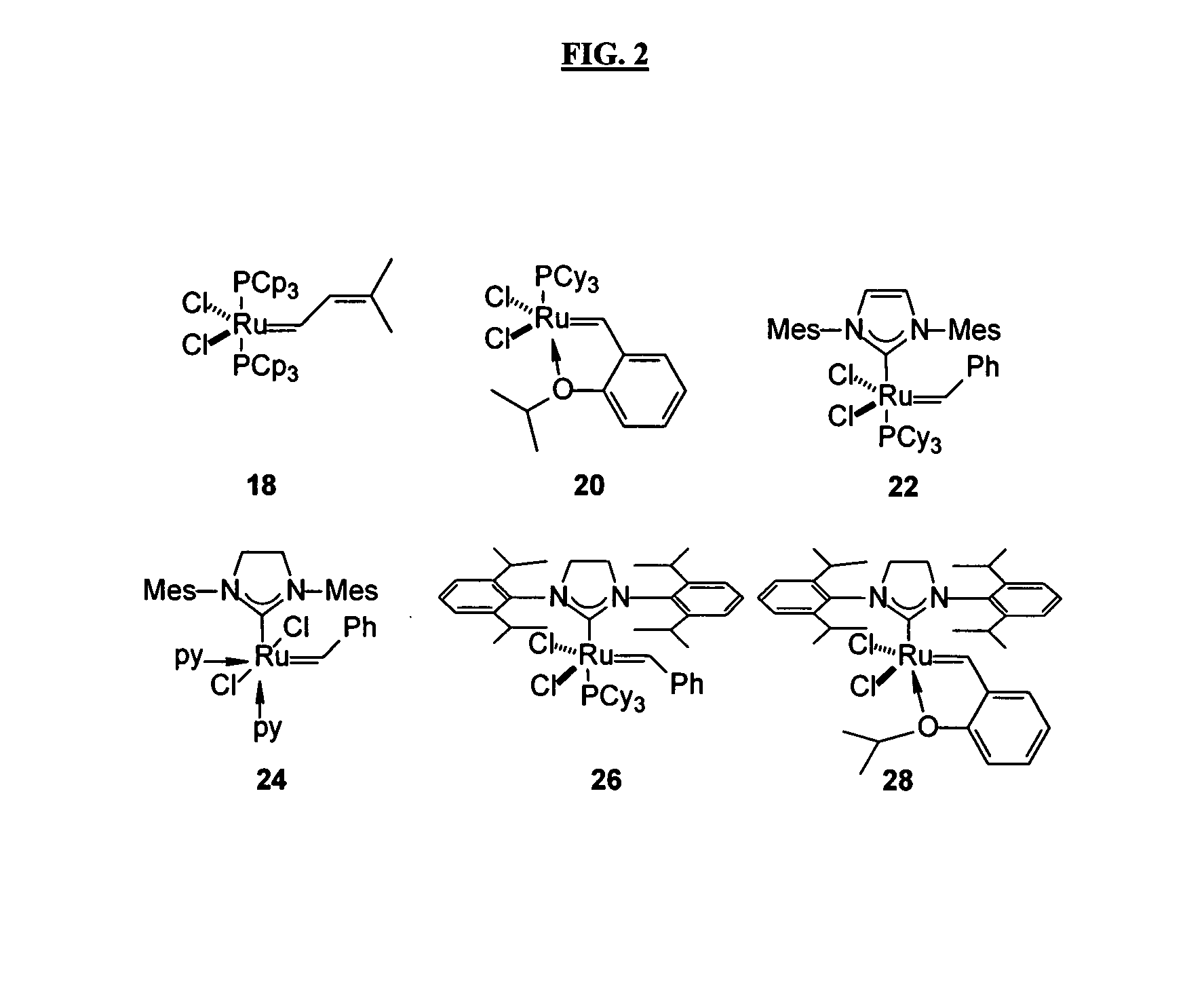
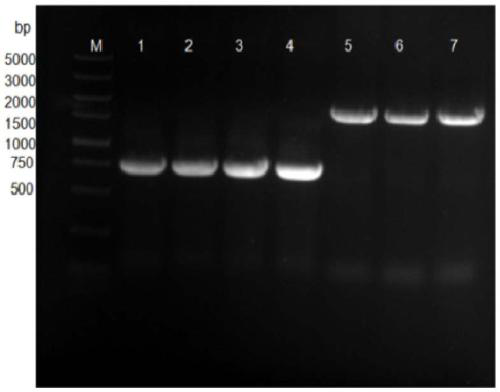
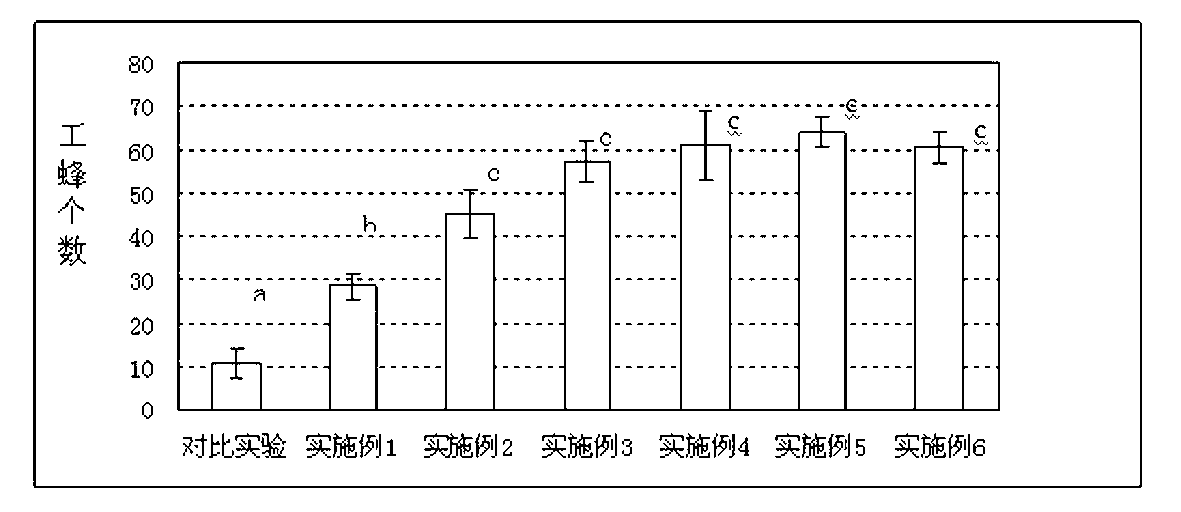
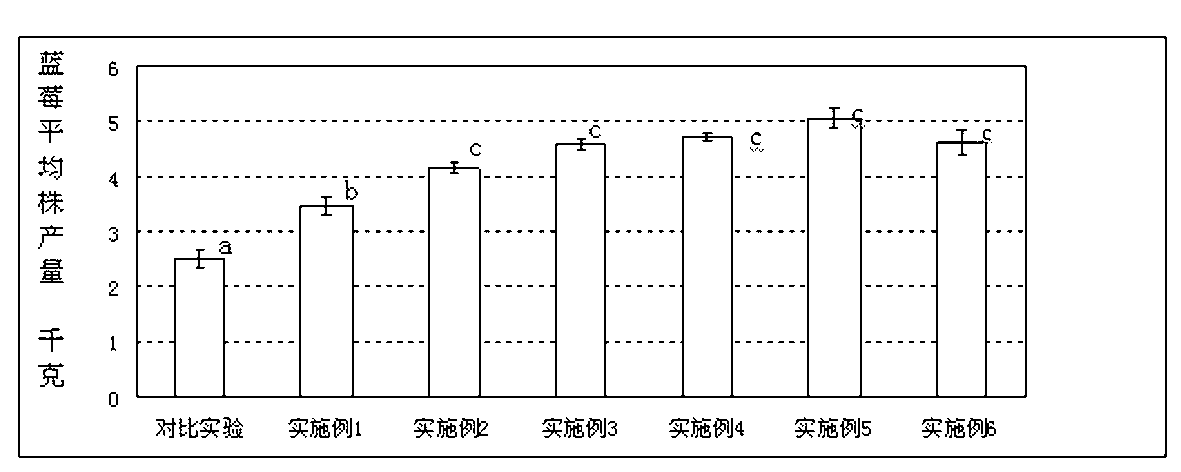
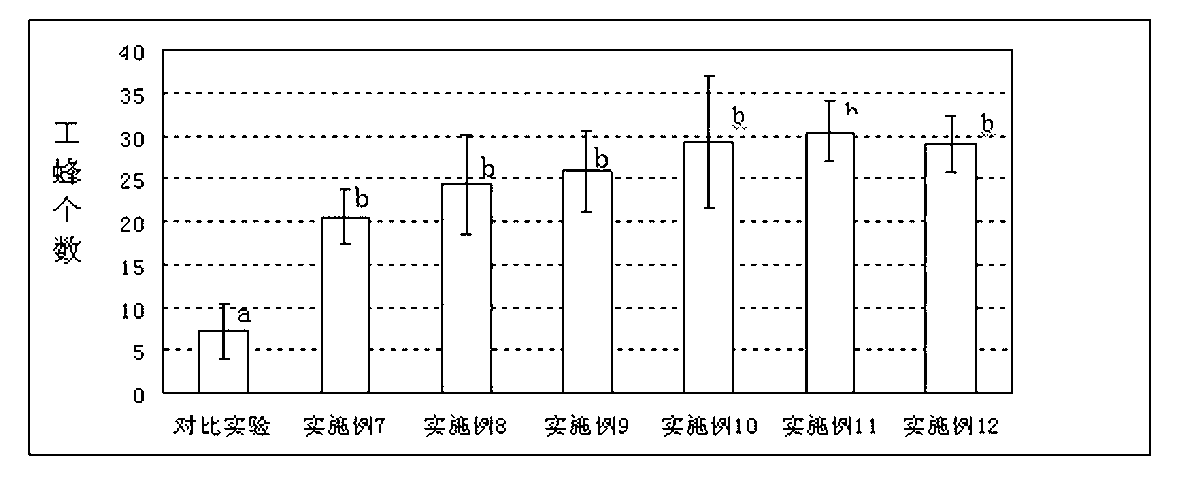
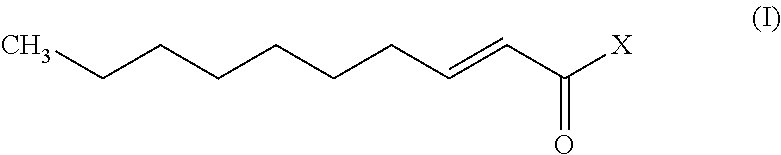
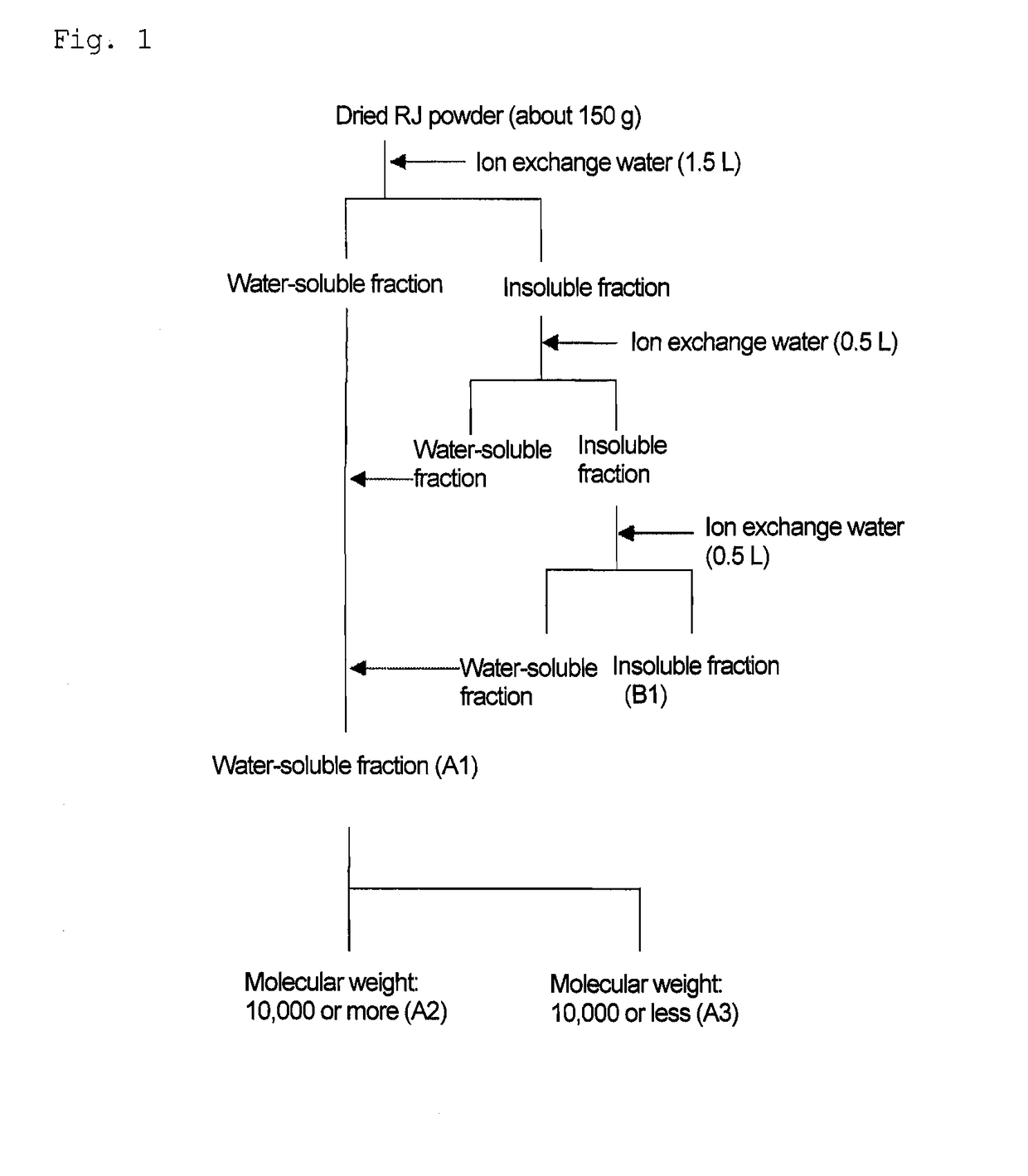
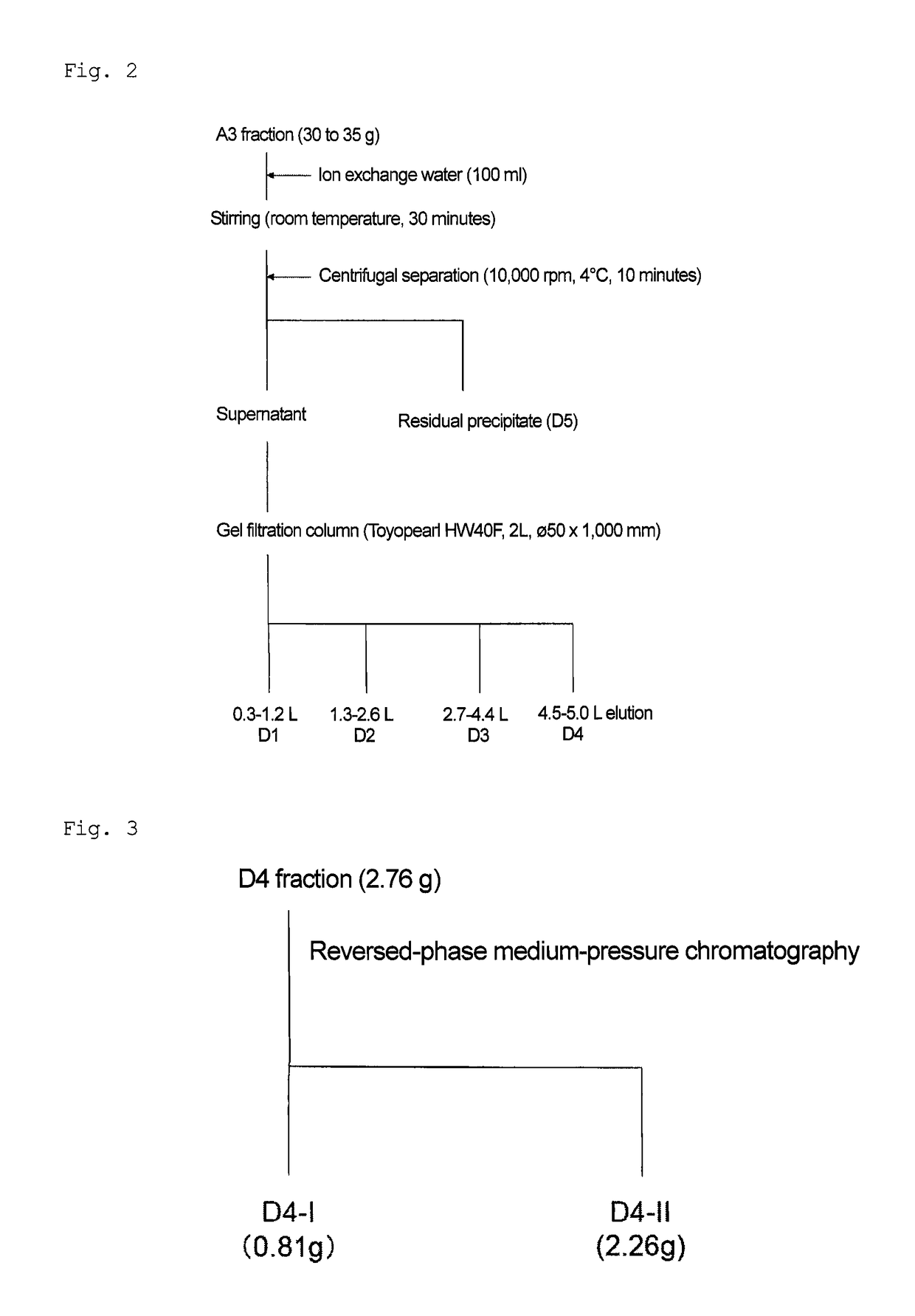
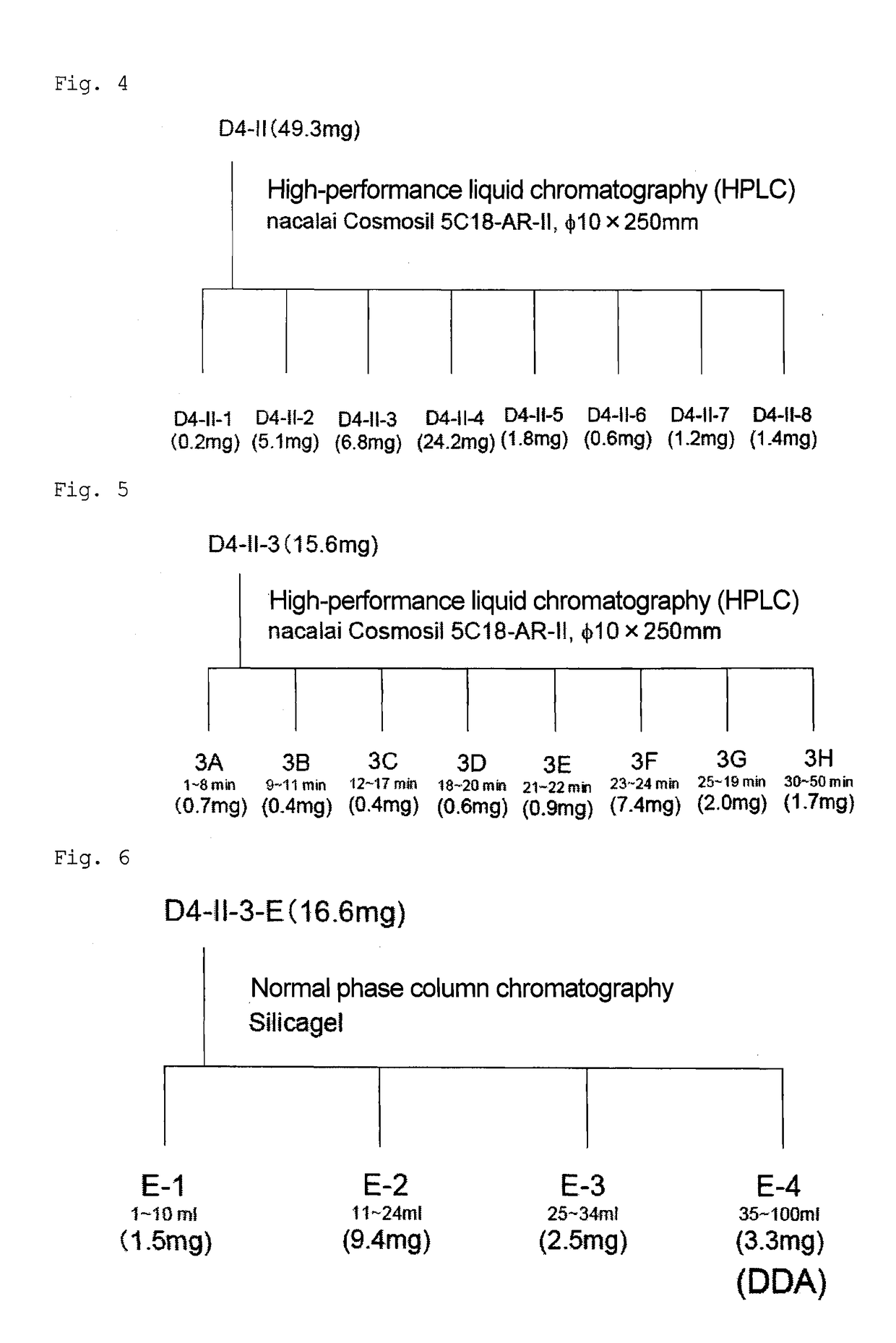
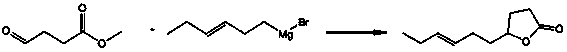Patents
Literature
70 results about "Decenoic Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
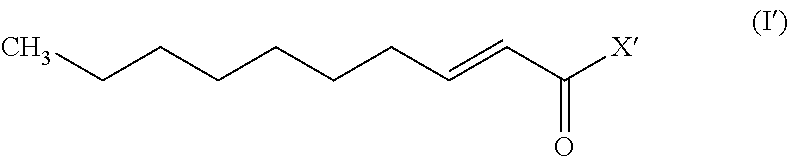
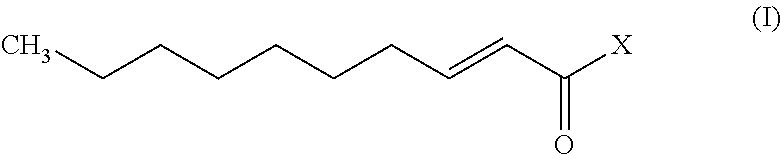
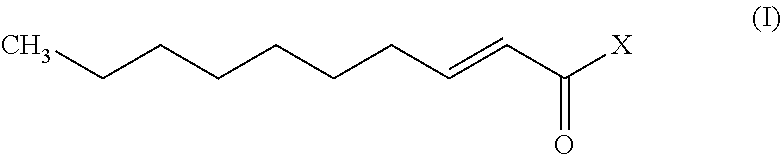
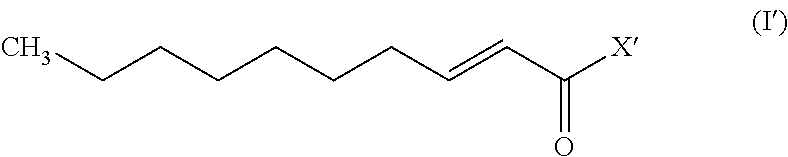
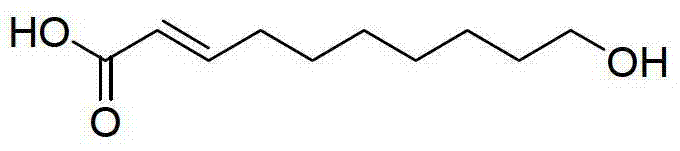
A monounsaturated medium-chain fatty acid with a 10-carbon backbone. Decenoic acid is found rarely in nature.
Antimicrobial compositions, methods and systems
InactiveUS20080033026A1Broad antimicrobial activity spectrumEfficacious activityBiocideDead animal preservationDecenoic AcidPHENOL LIQUID
The invention provides methods for treating a surface, the method including steps of applying a surface treatment composition to a surface, wherein the surface treatment composition includes a substantially phenol-free cleansing agent and an antimicrobial agent, the antimicrobial agent comprising 9-decenoic acid, a salt of 9-decenoic acid, an ester of 9-decenoic acid, or a combination thereof, wherein the antimicrobial agent is present in an amount sufficient to control microbial growth. Also described are methods for treating a surface that include the step of applying a surface treatment composition having a pH in the range of 4.1 to 8.5 to a surface, wherein the surface treatment composition includes a cleansing agent and an antimicrobial agent, the antimicrobial agent comprising 9-decenoic acid, a salt of 9-decenoic acid, an ester of 9-decenoic acid, or a combination thereof, wherein the antimicrobial agent is present in an amount sufficient to control microbial growth. Also described are surface treatment compositions including the antimicrobial agents.
Owner:ELEVANCE RENEWABLE SCIENCES INC
Natural antibacterial pearl wool
InactiveCN103333392AAvoid safety hazardsOvercome the disadvantages of prone to static electricityDecenoic AcidPolymer science
The invention relates to natural antibacterial pearl wool and belongs to the field of macromolecular materials. The pearl wool is characterized by comprising the following raw materials in parts by weight: 90 to 110 parts of low-density polyethylene (LDPE) resin particles, 1 to 2 parts of antistatic agent, 0.1 to 0.5 part of foaming agent, 0.001 to 0.005 part of color master batch, 0.3 to 0.6 part of monoglyceride, 0.5 to 1 part of calcium carbonate, 0.5 to 1 part of talc powder, 0.01 to 0.05 part of 10-hydroxy-2-decenoic acid, and 0.5 to 2 parts of melamine phosphate. By adopting the safe and non-flammable foaming agent, the antistatic agent and the flame retardant, the pearl wool has high safety factor; and by adding the natural antibacterial agent, the pearl wool has an antibacterial function.
Owner:SUZHOU NEW DISTRICT JIAHE PLASTIC
Novel Carboxylic Acid and Antidepressant Composition Containing the Same as Active Ingredient
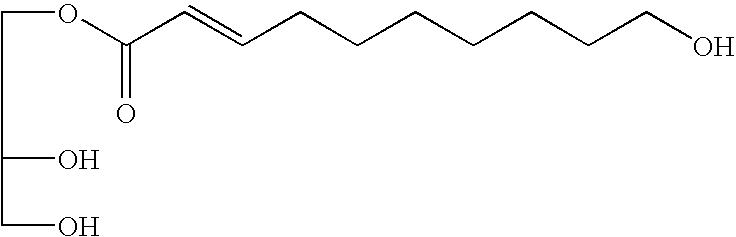
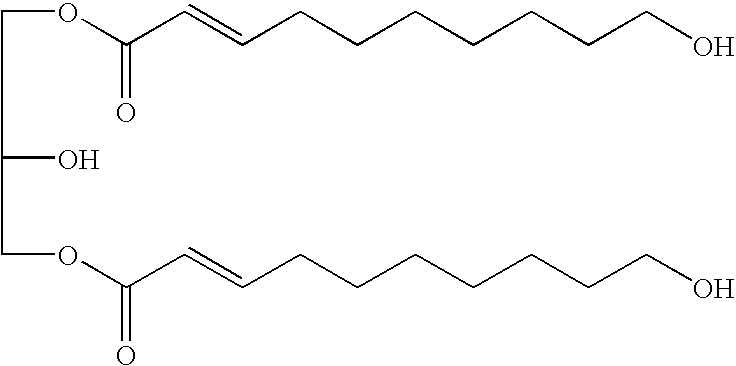
The present invention provides a novel compound and antidepressant composition that can be effectively used for improving depressed mood and depressed state, particularly for depressed mood and depressed state in menopausal women. The compound of the present invention is represented by the following formula (1):wherein R1 and R2 are identical or different and represent a hydrogen atom, a hydroxyl group or an acetyloxy group, and n is an integer of 2 to 7,or a pharmaceutically acceptable salt or ester thereof. This compound is used as an active ingredient in the antidepressant composition. Examples of the compound of the invention include (2E)-9,10-dihydroxy-2-decenoic acid, (2Z)-9,10-dihydroxy-2-decenoic acid, (2E)-9-hydroxy-2-decenoic acid, and (2E)-7-acetoxy-2-heptenoic acid.
Owner:YAMADA APICULTURE CENT
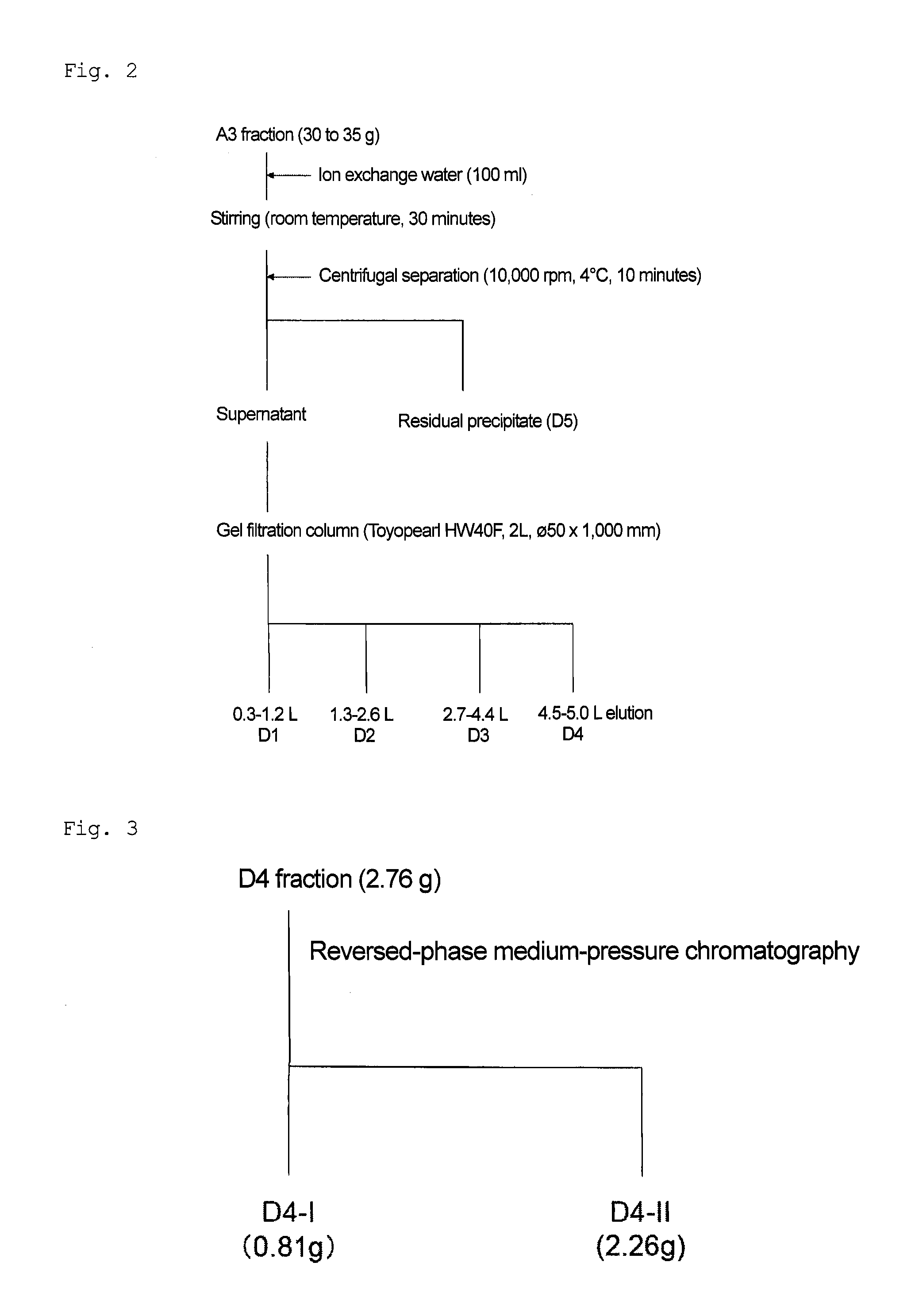
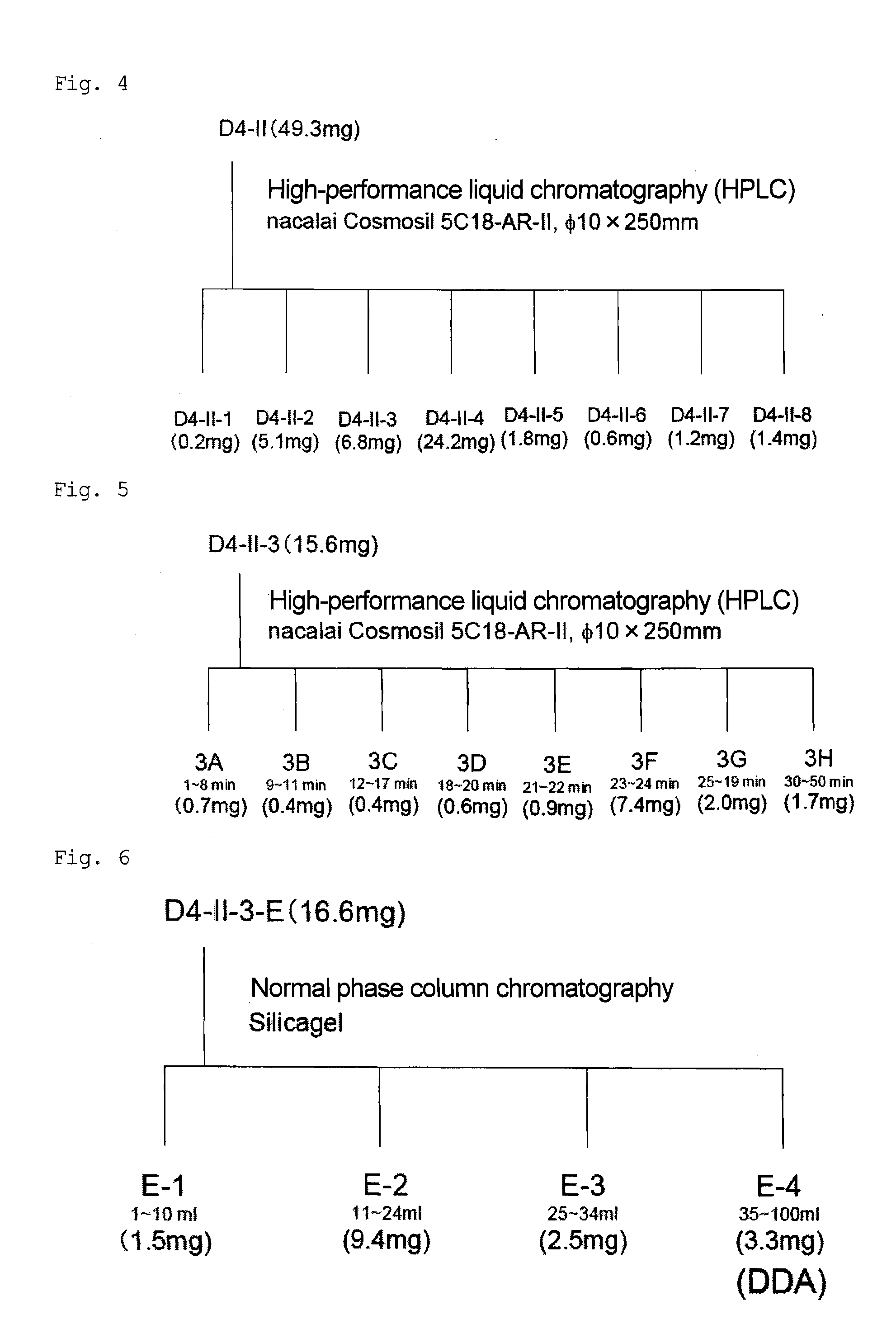
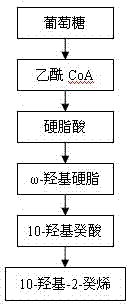
Method for preparing 10-hydroxyl-2-decenoic acid with decylic acid as raw material and by utilizing escherichia coli engineering bacteria
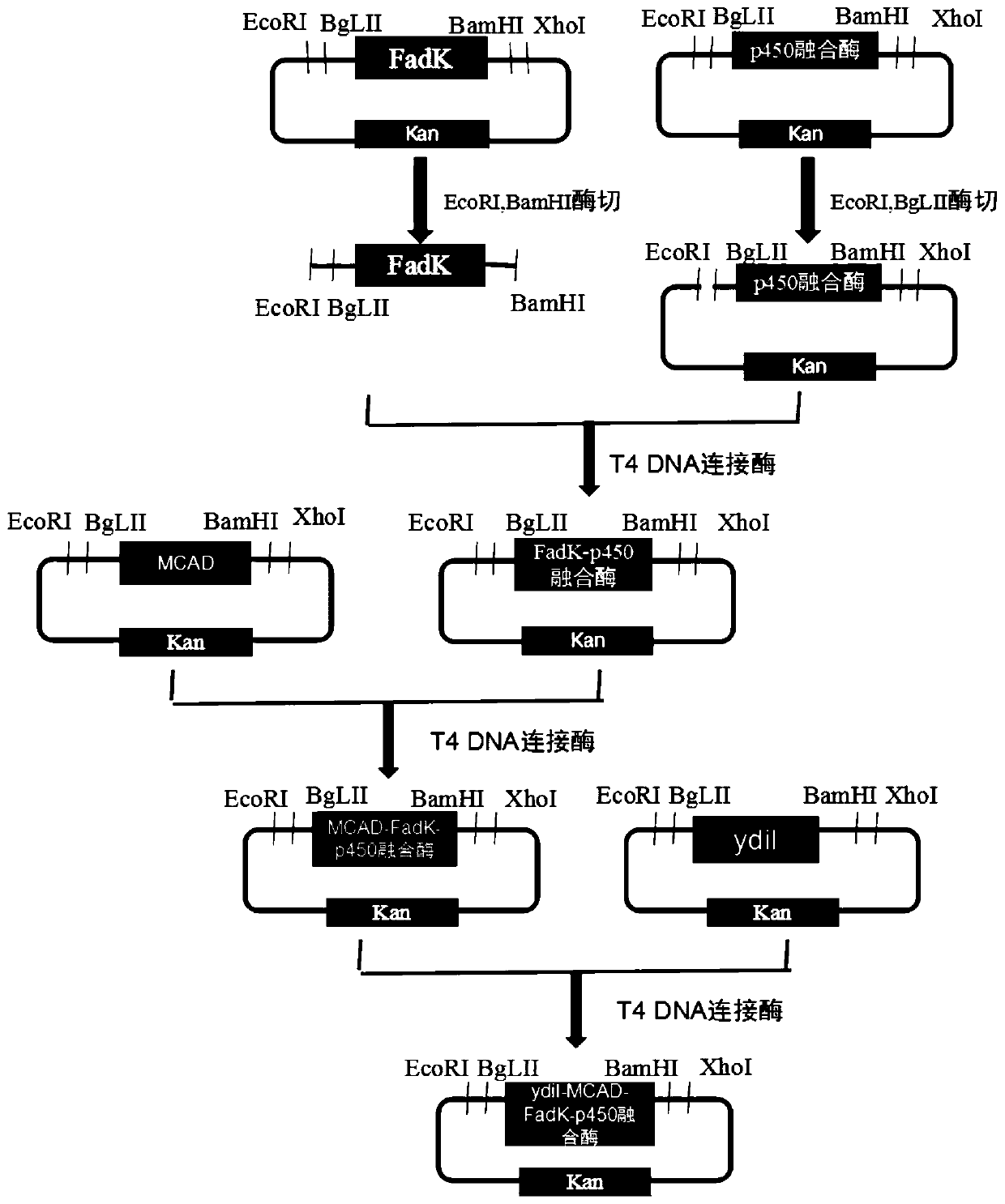
ActiveCN109897870AEfficient assemblyImprove conversion rateMicroorganism based processesFermentationDecenoic AcidEscherichia coli
The invention relates to a method for preparing 10-hydroxyl-2-decenoic acid by utilizing escherichia coli engineering bacteria. The method comprises the following steps that (1) a recombinant plasmidpBbB5K-P450 fusion enzyme, recombinant plasmids pBbB5K-FadK, recombinant plasmids pBbB5K-MCAD and recombinant plasmids pBbB5K-YdiI are constructed; (2) pBbB5K-ydiI-MCAD-FadK-P450 fusion enzyme recombinant plasmids are constructed; (3) the fusion enzyme recombinant plasmids are converted into escherichia coli, the escherichia coli are screened and subjected to induction culture, and thus inductioncells are prepared; and (4) the induction cells are cultured by a transformation culture medium, thus resting cells are prepared, then decylic acid is added into the culture medium, culturing is conducted, and thus the 10-hydroxyl-2-decenoic acid is prepared. The 10-hydroxyl-2-decenoic acid is produced in a fermented mode by utilizing the decylic acid as a raw material, and the process of producing the 10-hydroxyl-2-decenoic acid with the high added-value through the low-value decylic acid is realized.
Owner:QILU UNIV OF TECH
Method for preparing water soluble royal jelly
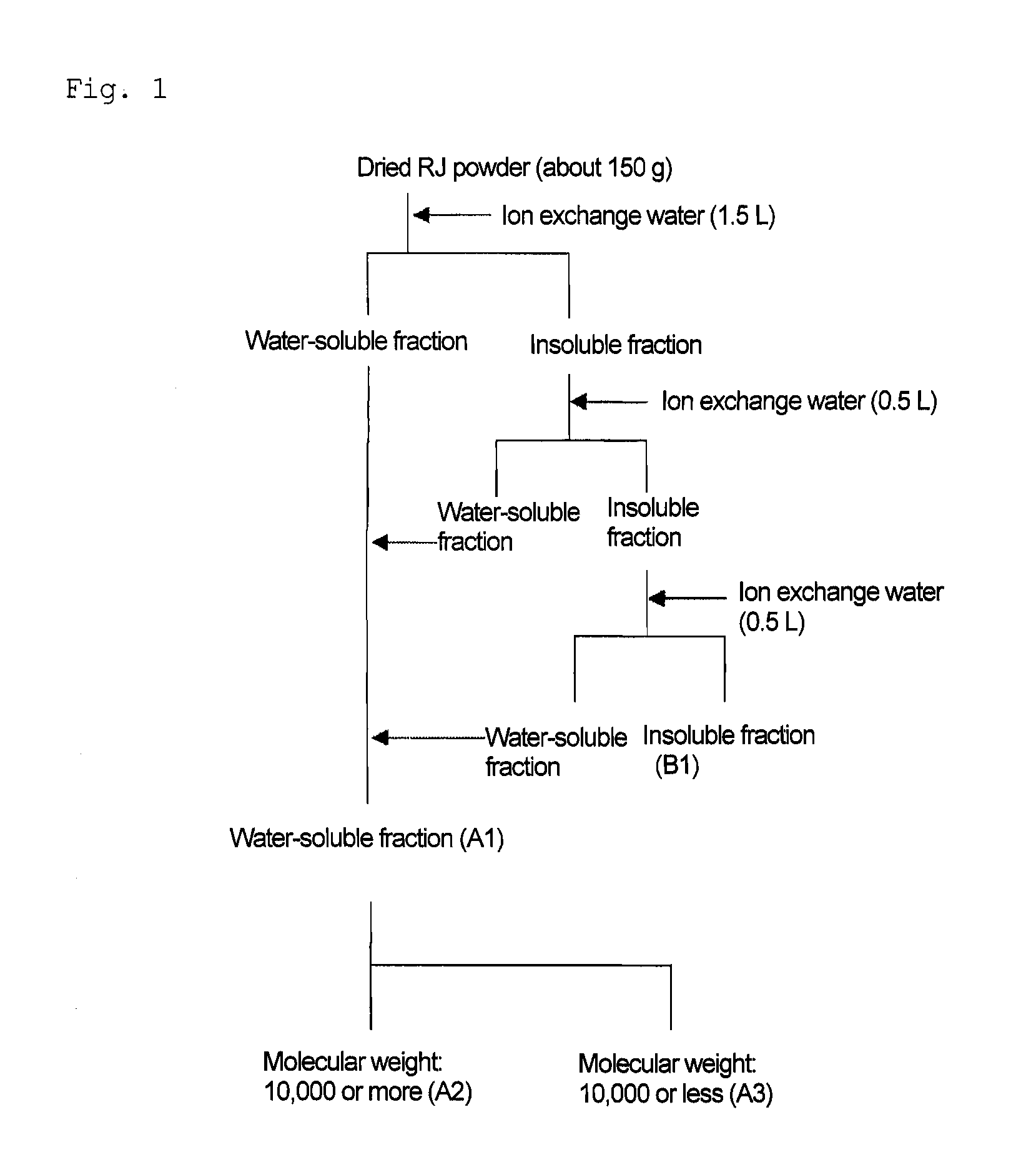
ActiveCN1899093AAvoid the influence of temperature-sensitive active substancesAvoid influenceFood preparationFreeze-dryingRoom temperature
The preparation process of water soluble royal jelly includes the following steps: defreezing royal jelly at room temperature; homogenizing via adding water in 1.5-3 times and stirring; enzymolyzing with proteinase at 40-45deg.c while stirring for 2-4 hr and ultrafiltering membrane or vacuum concentration at normal temperature to the amount of 1-1.5 times of royal jelly material; freeze drying; and vacuum packing. The water soluble royal jelly product has water content not more than 3 wt% and 10-HAD content not less than 4.0 wt%. The preparation process is simple and reliable, and the water soluble royal jelly product has wide dissolving temperature range, high stability, water dissolving retention rate as high as 90 % and other features.
Owner:杭州仟源保灵药业有限公司
Inductive production-increase agent for honeybee pollination on flowering plants
An inductive production-increase agent for the honeybee pollination on flowering plants comprises (E)-9-Oxodec-2-enoic acid (9-ODA), R (-)-9-hydroxy-(E)-2-decenoic acid (R(-)-9HDA), S(+)-9-hydroxy-(E)-2-decenoic acid (S (+) -9HDA), Methyl 4-hydroxybenzoate (HOB), 4-hydroxy-3-methoxyphenylacetic (HVA), 9-Octadecenoic acid methyl ester (methyl oleate), (E)-4-(4-Hydroxy-3-methoxypheny)-3-2-propen-1-ol (coniferyl alcohol), hexadecanol and (Z9, Z12, Z15)-9, 12, 15-octadecatrienoic acid (linolenic acid); the invention can effectively induce a honeybee to pollinate on flowering plants. The inductive production-increase agent has a positive effect on the production increase of honeybee pollination on blueberries and cherries; it effectively extrudes the application of the queen bee flowing element in the inductive production increase in the pollination on blueberries and cherries.
Owner:ZHEJIANG FENGMEI BIOLOGICAL SCI & TECH
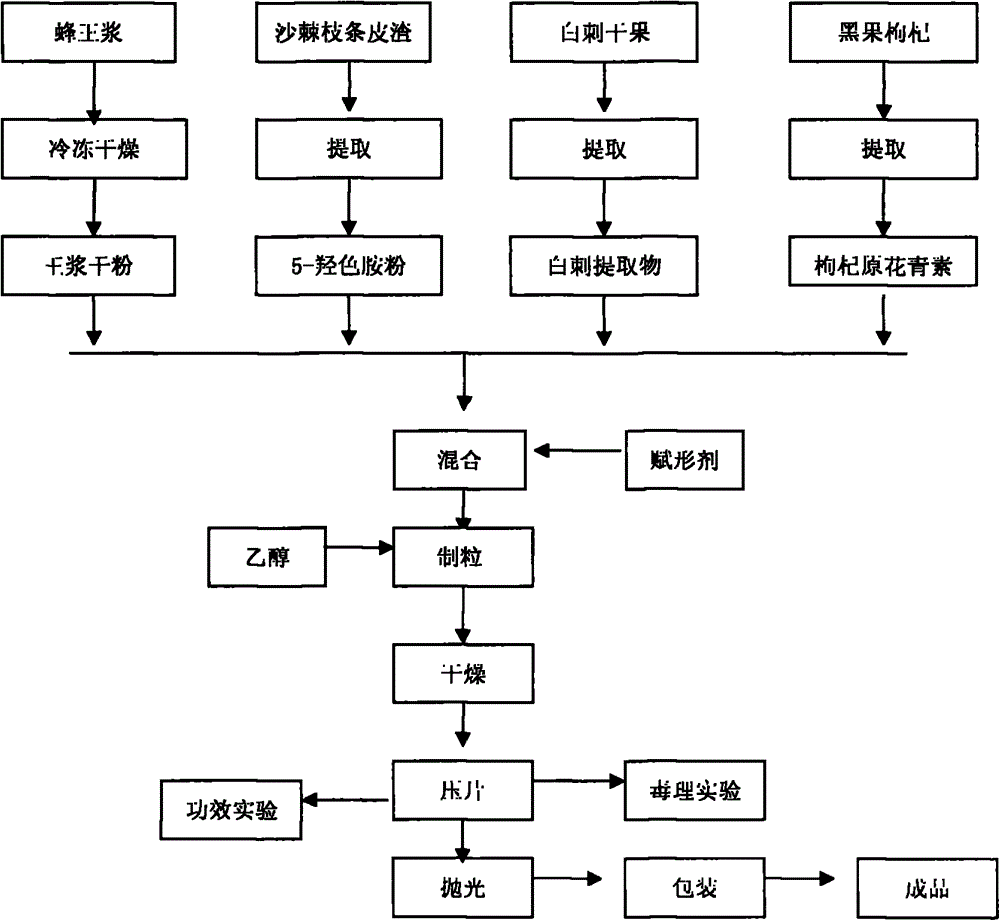
Plateau royal jelly berry buccal tablet and preparation method thereof
ActiveCN104544041AReduce depressionImprove sleepingFood shapingNatural extract food ingredientsDecenoic AcidNitraria sibirica
The invention relates to a plateau royal jelly berry buccal tablet and a preparation method thereof. The product is prepared by extracting 10-hydroxy-2-decenoic acid, 5-hydroxytryptamine and procyanidine from royal jelly, seabuckthorn fruit, nitraria sibirica pall and lycium ruthenicum murr, granulating, drying, arranging granules and tabletting. The invention aims at providing a plateau royal jelly berry buccal tablet which is rich in 10-hydroxy-2-decenoic acid, 5-hydroxytryptamine and procyanidine; the buccal tablet has functions of promoting sleeping, relieving the occurrence of depression, resisting oxidation and delaying aging, and the buccal tablet is broad in market prospect.
Owner:GEERMU YUANXINTANG BIOLOGICAL SCI & TECH
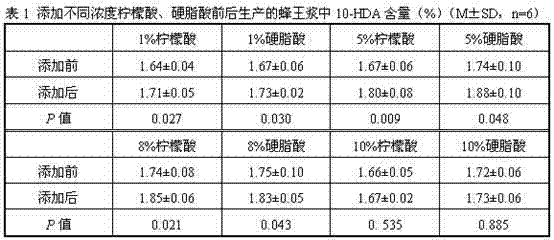
Method for improving 10-hydroxy-2-caproleic acid content of royal jelly
ActiveCN102415508AMeeting nutritional needsIncrease 10-HDA contentAnimal feeding stuffBiotechnologyDecenoic Acid
The invention provides a method for improving 10-hydroxy-2-caproleic acid content of royal jelly. The method comprises the steps of adding 1 to 8% of citric acid or eleaostearic acid into pollen forage for supplying protein nutrients to bees, and continuously feeding bees with the pollen forage with citric acid or eleaostearic acid for 9 days. The method is designed according to a bee biology principle and a synthesis approach of 10-HDA in bees, is in accord with a biology principle, satisfies nutrition demands from bees, can obviously improve 10-HDA content of royal jelly, utilizes common food-grade edible substances as additives, does not produce food safety problems, has a short feeding period, simple operation processes and short onset time, and can be utilized in preparation of 10-hydroxy-2-caproleic acid-containing royal jelly.
Owner:ZHEJIANG UNIV
An application of E-10-hydroxy-2-decenoic acid in preparation of medicines or healthcare products for hepatic disease
PendingCN104825435ASolve usabilitySolve complexityOrganic active ingredientsDigestive systemDecenoic AcidHepatic lipase
The invention discloses an application of E-10-hydroxy-2-decenoic acid in preparation of medicines or healthcare products for hepatic disease, and relates to the technical field of applications of hydroxy-containing unsaturated compounds in which carboxyl is connected to a non-ring carbon. The hepatic disease relates to fatty liver, hepatitis or liver cirrhosis. The E-10-hydroxy-2-decenoic acid has definite treatment or aided treatment functions for the hepatic disease, can be used for preparation of the medicines or healthcare products which are used for treatment or aided treatment of the hepatic disease, and has advantages of low cost, good effects, low using amount, and no side or toxic effects.
Owner:SHIJIAZHUANG KANGNUO BIOTECH
Use of at least one 10-hydroxy-2-decenoic acid derivative in compositions for treating the cuteous signs of aging
The present invention relates to a method of inhibiting degradation of skin or mucous membranes by inhibiting action of collagenases comprising applying to the skin or mucous membranes a composition comprising at least one of 10-hydroxy-2-decenoic acid derivative. The invention also relates to a process for treating signs of aging.
Owner:LOREAL SA
L plantarum LP45 living bacterium preparation for regulating intestinal flora and application
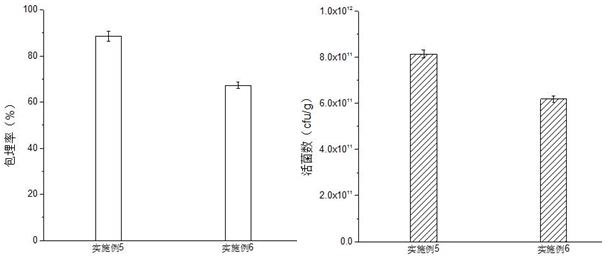
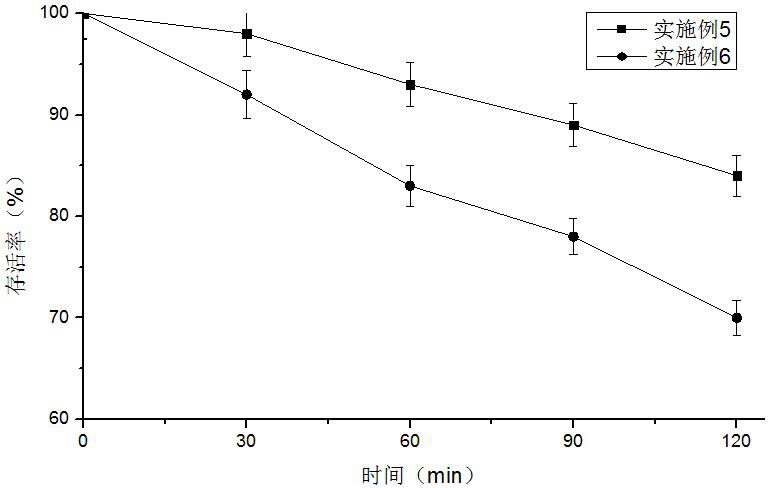
ActiveCN112094790AImprove protectionFree from damagePowder deliveryBacteriaBiotechnologyDecenoic Acid
The invention provides an L plantarum living bacterium preparation and an application thereof, and belongs to the technical field of biology. The L plantarum living bacterium preparation comprises freeze-dried L plantarum powder and freeze-dried L plantarum microcapsules, wherein a freeze-drying protective agent used in the preparation process comprises 1-3wt% of glycerol and 0.05-0.15 wt% of 10-hydroxyl-2-decenoic acid, and the mass ratio of the 10-hydroxy-2-decenoic acid to the glycerol is 1: (15-20). The L plantarum viable bacterium preparation provided by the invention has the advantages of high biological activity, capability of enhancing the body immune function and capability of efficiently and durably adjusting intestinal flora balance.
Owner:河北一然生物科技股份有限公司
Process for extracting 10-oxhydryl-2-caproleic acid from royal jelly
InactiveCN103044240AMild conditionsHigh recovery rateCarboxylic compound separation/purificationDecenoic AcidDesorption
Owner:SHANDONG HUAKANG HONEY PROD
Mutant enzyme CYP153A M228L and application thereof in synthesis of 10-hydroxy-2-decenoic acid
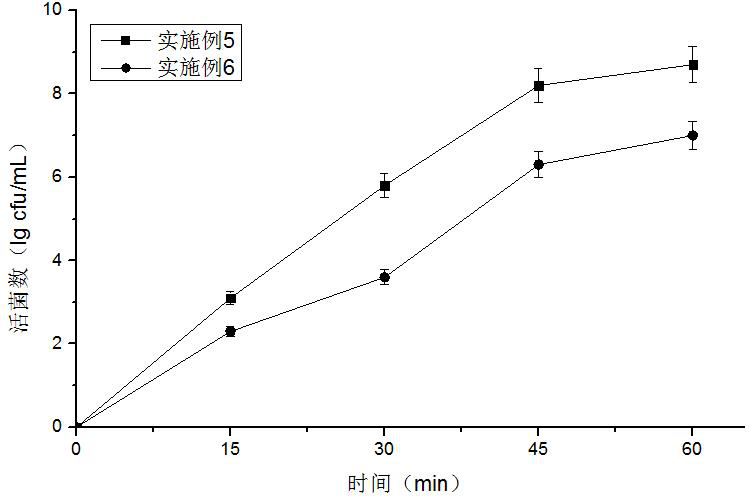
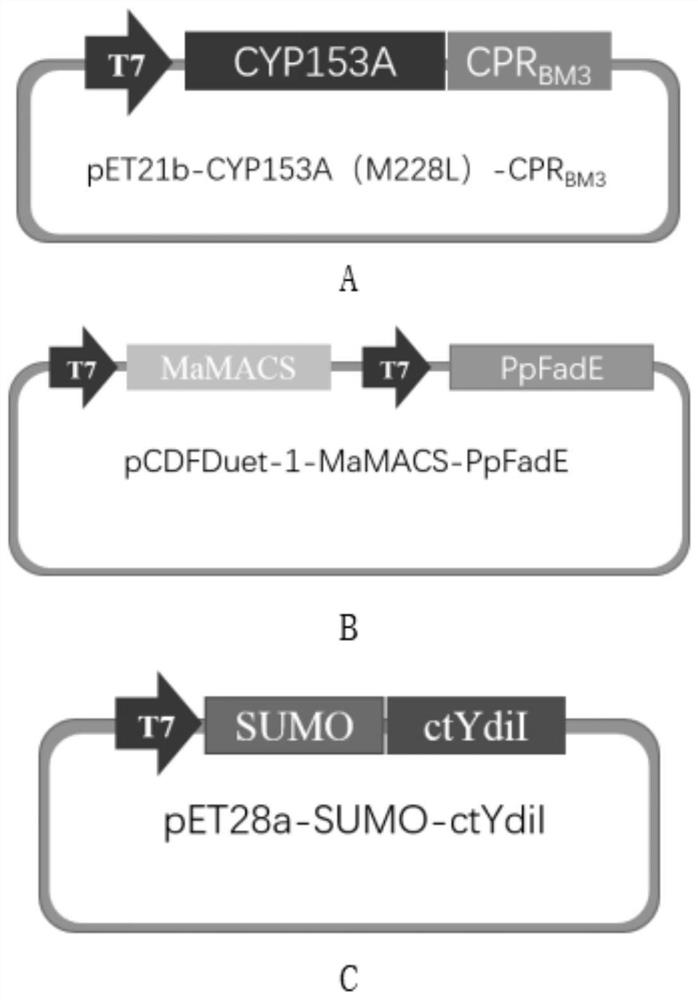
The invention relates to a mutant enzyme CYP153A M228L and an application of the mutant enzyme CYP153A M228L in synthesis of 10-hydroxy-2-decenoic acid. The mutant enzyme CYP153A M228L is characterized in that amino acid at the 228th site of CYP153A enzyme is mutated into L from M; a method for biologically synthesizing 10-hydroxy-2-decenoic acid through a two-step method by taking decanoic acid as a raw material mainly comprises the following steps: constructing an optimized recombinant plasmid pCDFDuet-1-MaMACS-PpFadE, an optimized recombinant plasmid pET21b-CYP153A M228L-CPRBM3, and an optimized recombinant plasmid pET28a-SUMO-ctYdiI; constructing escherichia coli recombinant bacteria to prepare resting cells, further culturing the resting cells to prepare the 10-hydroxy-2-decenoic acid. The conversion rate of the 10-hydroxy-2-decenoic acid is remarkably improved according to the technical scheme.
Owner:QILU UNIV OF TECH
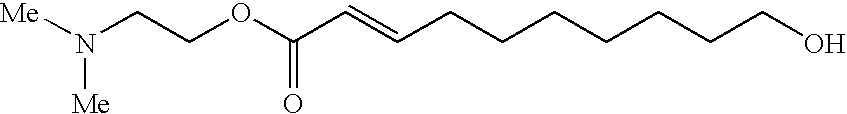
Trans-2-decenoic acid derivative and pharmaceutical agent containing the same
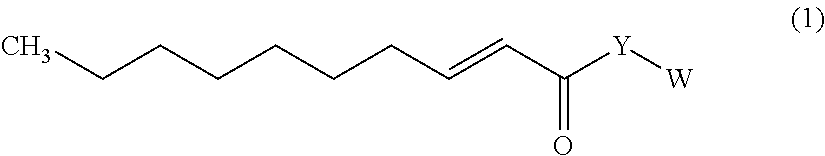
ActiveUS20130225837A1Excellent neurotrophic factor-like activityHighly safe preventiveNervous disorderFatty acid chemical modificationDecenoic AcidAnticarcinogen
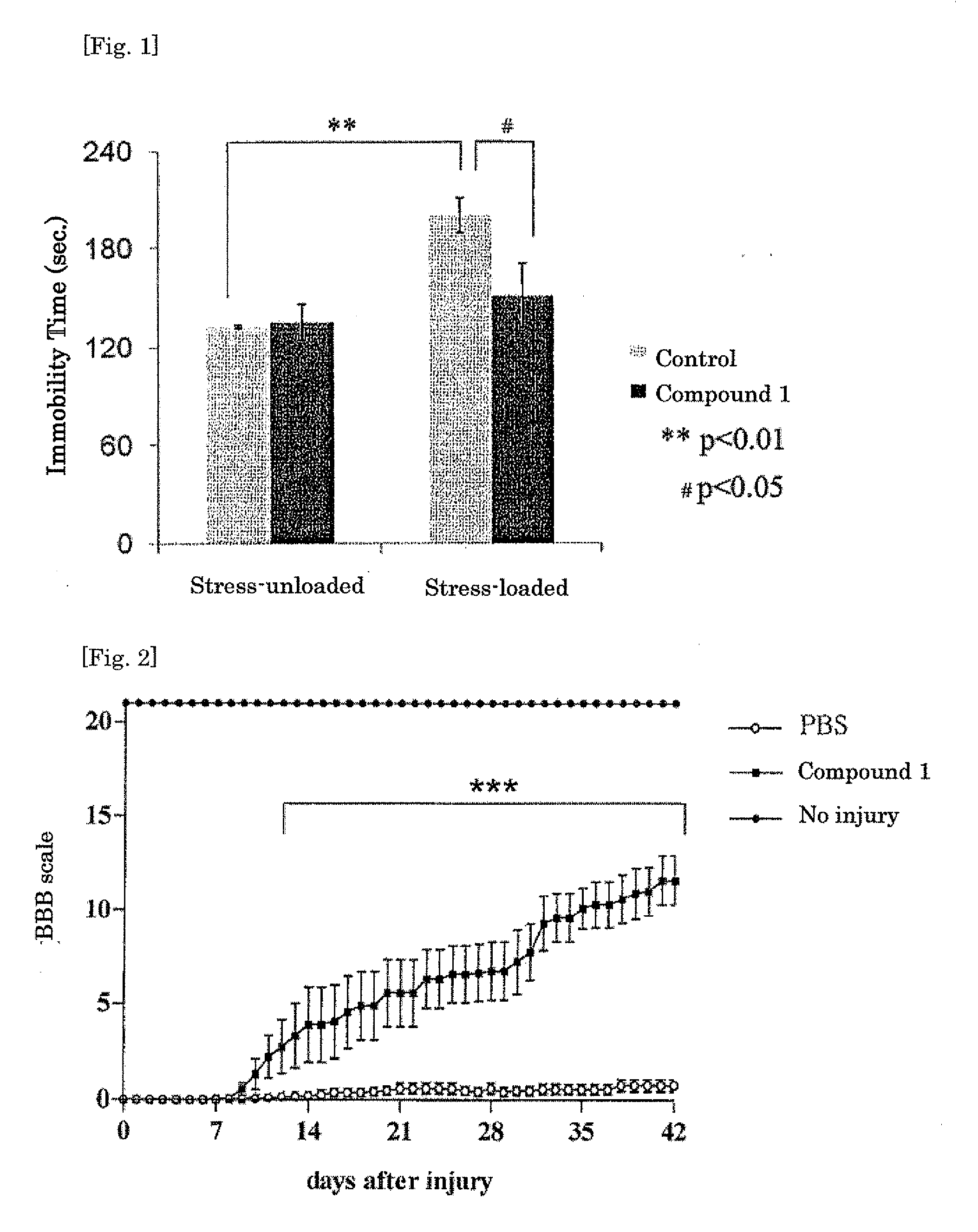
An object of the present invention is to provide a novel trans-2-decenoic acid derivative or a pharmaceutically acceptable salt thereof and to provide a pharmaceutical agent which contains said compound as an active ingredient and has a highly safe neurotrophic factor-like activity or an alleviating action for side effect induced by administration of anti-cancer agents. The trans-2-decenoic acid derivative or a pharmaceutically acceptable salt thereof which is the compound of the present invention is specifically represented by the formula (1):(In the formula, Y is —O—, —NR— or —S—, R is hydrogen atom, alkyl group, dialkylaminoalkyl group or the like and W is a substituent such as dialkylaminoalkyl group) and has a quite high usefulness as a pharmaceutical agent such as a preventive or therapeutic agent for dementia, Alzheimer's disease, Parkinson's disease, depression, etc., a treating or repairing agent for spinal cord injury.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
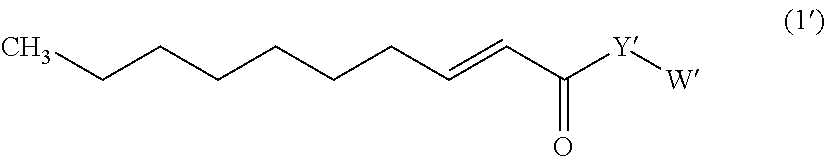
Trans-2-decenoic acid derivative and drug containing same
ActiveUS20150087823A1Prevention or treatment of nerve disordersEffective preventionSenses disorderNervous disorderDecenoic AcidAnticarcinogen
The present invention relates to a novel trans-2-decenoic acid derivative or a pharmaceutically acceptable salt thereof, and a pharmaceutical agent containing the compound as an active ingredient. The trans-2-decenoic acid derivative or a pharmaceutically acceptable salt, which is the compound of the present invention, is specifically represented by the general formula (I):wherein X is a substituent such as a 1-pyrrolidyl, a 3-thiazolizyl, or a piperidino, and the compound is highly useful as a pharmaceutical agent, such as a prophylactic or therapeutic agent for a peripheral nerve disorder induced by administration of an anticancer agent, a prophylactic or therapeutic agent for neurodegenerative diseases or mental diseases such as dementia, Alzheimer's disease, Parkinson's disease, diabetic neuropathy, depression, glaucoma, or autistic disorder spectrum, a therapeutic or repairing agent for spinal cord injury, analgesics against various pain diseases, or the like.
Owner:NIPPON ZOKI PHARM CO LTD +1
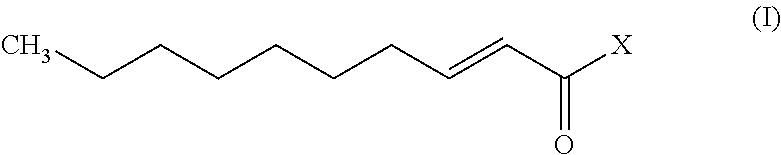
Trans-2-decenoic acid derivative and drug containing same
ActiveUS9428477B2Prevention or treatment of nerve disordersEffective preventionSenses disorderNervous disorderDecenoic AcidAnticarcinogen
Owner:NIPPON ZOKI PHARM CO LTD +1
Method for extracting 10-hydroxy-2-decenoic acid from royal jelly freeze-dried powder
InactiveCN107641077AImprove hydrophobicityShorten the total process timeCarboxylic compound separation/purificationAcetic acidDecenoic Acid
The embodiment of the invention provides a method for extracting 10-hydroxy-2-decenoic acid from royal jelly freeze-dried powder. The method comprises the following steps: (1) dispersing the royal jelly freeze-dried powder into water and stirring to obtain a dispersion solution; (2) regulating the pH (Potential of Hydrogen) value of the dispersion solution to 2 to 3; centrifuging and taking sediment; (3) adding the sediment into ethyl acetate and stirring; centrifuging and taking supernatant; (4) decompressing and distilling the supernatant to obtain a product containing the 10-hydroxy-2-decenoic acid. The extraction method provided by the invention has a simple technology and short time consumption; the content of the 10-hydroxy-2-decenoic acid in the obtained product is high and the product can meet application to development of medicines and health-care foods.
Owner:刘峰
Carboxylic acid and antidepressant composition containing the same as active ingredient
The present invention provides a novel compound and antidepressant composition that can be effectively used for improving depressed mood and depressed state, particularly for depressed mood and depressed state in menopausal women. The compound of the present invention is represented by the following formula (1):wherein R1 and R2 are identical or different and represent a hydrogen atom, a hydroxyl group or an acetyloxy group, and n is an integer of 2 to 7,or a pharmaceutically acceptable salt or ester thereof. This compound is used as an active ingredient in the antidepressant composition. Examples of the compound of the invention include (2E)-9,10-dihydroxy-2-decenoic acid, (2Z)-9,10-dihydroxy-2-decenoic acid, (2E)-9-hydroxy-2-decenoic acid, and (2E)-7-acetoxy-2-heptenoic acid.
Owner:YAMADA APICULTURE CENT
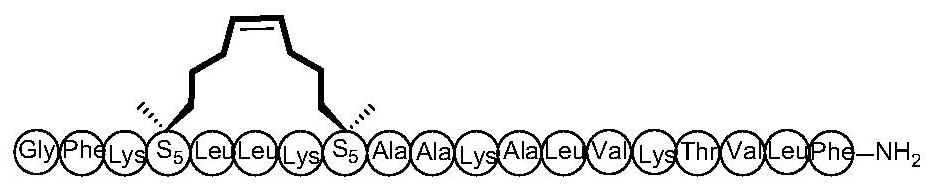
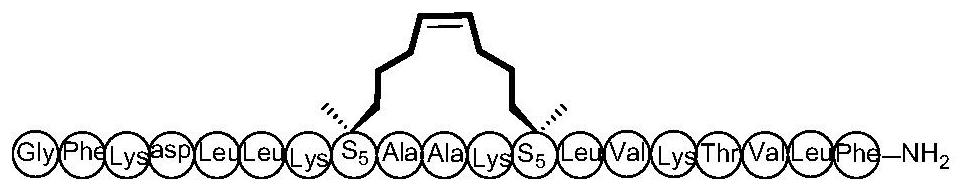
Cyclopeptide antitumor active compound and preparation method and application thereof
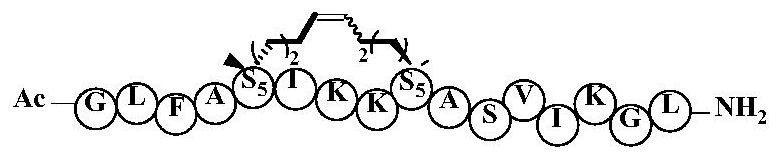
ActiveCN112778403AEnhanced alpha helical configurationEnhanced α-helical structurePeptidesCyclic peptide ingredientsCyclic peptideDecenoic Acid
The invention relates to the technical field of medicines, and discloses a cyclopeptide antitumor active compound, and particularly relates to a cyclopeptide active molecule with a structure shown in a formula (I), the cyclopeptide active molecule with the structure shown in the formula (I) and pharmaceutically acceptable salt or ester thereof: GFKX1LLKX2X3AKX4LVKX5VLF.NH2(I), wherein X1 represents aspartic acid or (2R)-2-amino-2-methyl-6-heptenoic acid; X2 represents glycine or (2R)-2-amino-2-methyl-6-heptenoic acid; X3 represents alanine or (2R)-2-amino-2-methyl-9-decenoic acid; X4 represents alanine or (2R)-2-amino-2-methyl-6-heptenoic acid; X5 represents threonine or (2R)-2-amino-2-methyl-6-heptenoic acid; and paired (2R)-2-amino-2-methyl-6-heptenoic acid or (2R)-2-amino-2-methyl-9-decenoic acid in a fragment is cyclized through an olefin metathesis reaction. The cyclopeptide antitumor active compound and a preparation method and application thereof aim to enhance the cell permeability and improve the enzyme stability and antitumor activity.
Owner:SHANGHAI UNIV
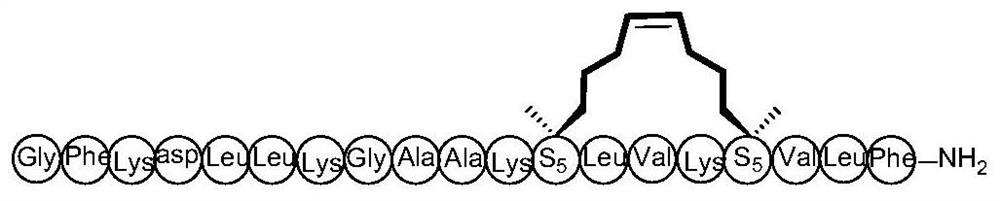
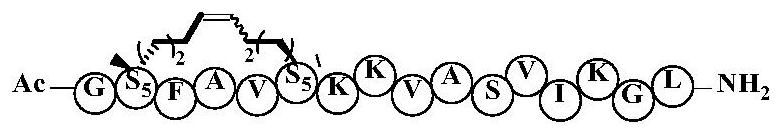
Cyclic peptide anti-tumor activity compound and preparation method therefor and application of cyclic peptide anti-tumor activity compound
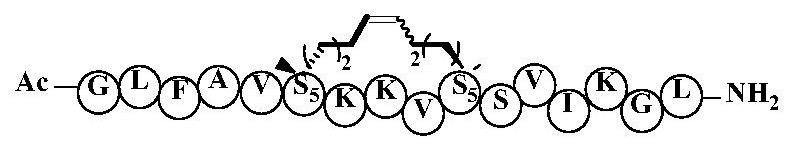
PendingCN111662363AEnhanced alpha helical configurationImprove permeabilityPeptidesCyclic peptide ingredientsCyclic peptideDecenoic Acid
The invention relates to the technical field of medicine, and discloses a cyclic peptide anti-tumor activity compound, in particular to a cyclic peptide active molecule with a structure shown by a formula (I). According to the cyclic peptide active molecule with the structure of the formula (I) and pharmaceutically acceptable salt or ester thereof: GX1FAX2X3KKX4ASVX5KX6L (I), X1 represents leucineor (2R)-2-amino-2-methyl-6-heptenoic acid or (2R)-2-amino-2- methyl-9-decenoic acid; X2 represents valine or (2R)-2-amino-2-methyl-6-heptenoic acid; X3 represents isoleucine or (2R)-2-amino-2-methyl-6-heptenoic acid; X4 represents valine or (2R)-2-amino-2-methyl-6-heptenoic acid; X5 represents isoleucine or (2R)-2-amino-2-methyl-6-heptenoic acid; X6 represents glycine or (2R)-2-amino-2-methyl-6-heptenoic acid; and coupled (2R)-2-amino-2-methyl6-heptenoic acids or (2R)-2-amino-2-methyl-9-decenoic acids in a fragment are cyclized through olefin metathesis. The cyclic peptide anti-tumor activitycompound and a preparation method therefor and application of the cyclic peptide anti-tumor activity compound aim to enhance the cell permeability of the cyclic peptide anti-tumor activity compound,and to improve enzyme stability and anti-tumor activity.
Owner:SHANGHAI UNIV
Substrate for medical adhesive tapes and preparation method thereof
The invention relates to a substrate for medical adhesive tapes and a preparation method thereof and belongs to the technical field of medical materials. The substrate for medical adhesive tapes comprises the following components in parts by weight: 20-32 parts of polyisobutylene, 12-18 parts of PET resin, 5-10 parts of glass fiber, 1-3 parts of 10-hydroxy-2-decenoic acid, 6-12 parts of nano silicon dioxide, 1-4 parts of tri[2,4-di-tert-butylphenyl]phosphate, 7-15 parts of a citric acid, 1-5 parts of zinc stearate, 2-5 parts of tri-n-butyl citrate, and 4-10 parts of polyethylenimine. The substrate for medical adhesive tapes disclosed by the invention has excellent air permeability, and if a medical adhesive tape is in contact with the skin, the medical adhesive tape can keep the skin refreshing and ventilated; the substrate for medical adhesive tapes disclosed by the invention can endow an adhesive tape with good tearability, so that the adhesive tape is easy to use; the substrate for medical adhesive tapes disclosed by the invention has good antibacterial properties; and fourthly, the preparation method disclosed by the invention is simple and practicable, convenient to implement, and suitable for large-scale popularization and application.
Owner:SUZHOU QISHUO INFORMATION TECH CO LTD
Process for extracting 10-hydroxy-2-decenoic acid from royal jelly waste
InactiveCN105712872ALow costSimple preparation processCarboxylic compound separation/purificationDecenoic AcidFiltration
The invention discloses a process method for extracting 10-hydroxy-2-decenoic acid from royal jelly waste, wherein the process method comprises the following steps: (1) mixing the royal jelly waste with 95% edible alcohol according to the weight ratio of 1 to (8-10), adding the mixture into a reaction kettle, carrying out heating reflux for 40 minutes, then filtering while being hot, adding edible alcohol into the royal jelly waste, repeatedly extracting for 2-3 times, filtering, merging the filtrates, and carrying out reduced pressure recycling of ethyl alcohol; (2) concentrating the filtrate to 7-8 L, adding distilled water, heating to be transparent with steam, carrying out static filtration, washing the obtained crystal for three times with distilled water, and drying at the temperature of 50 DEG C, to obtain a crude product; and (3) adding a 50% methanol aqueous solution with the volume two times that of the crude product into the crude product, and recrystallizing for 3-4 times to obtain a 10-hydroxy-2-decenoic acid refined product. The process is simple, the cost is low, the 10-hydroxy-2-decenoic acid is extracted from the royal jelly waste, the maximum utilization rate of resources is achieved, the purity of the obtained 10-hydroxy-2-decenoic acid refined product reaches more than or equal to 99%, and the 10-hydroxy-2-decenoic acid can be used for production of antibacterial, anti-cancer and anti-radiation drugs.
Owner:刘春香
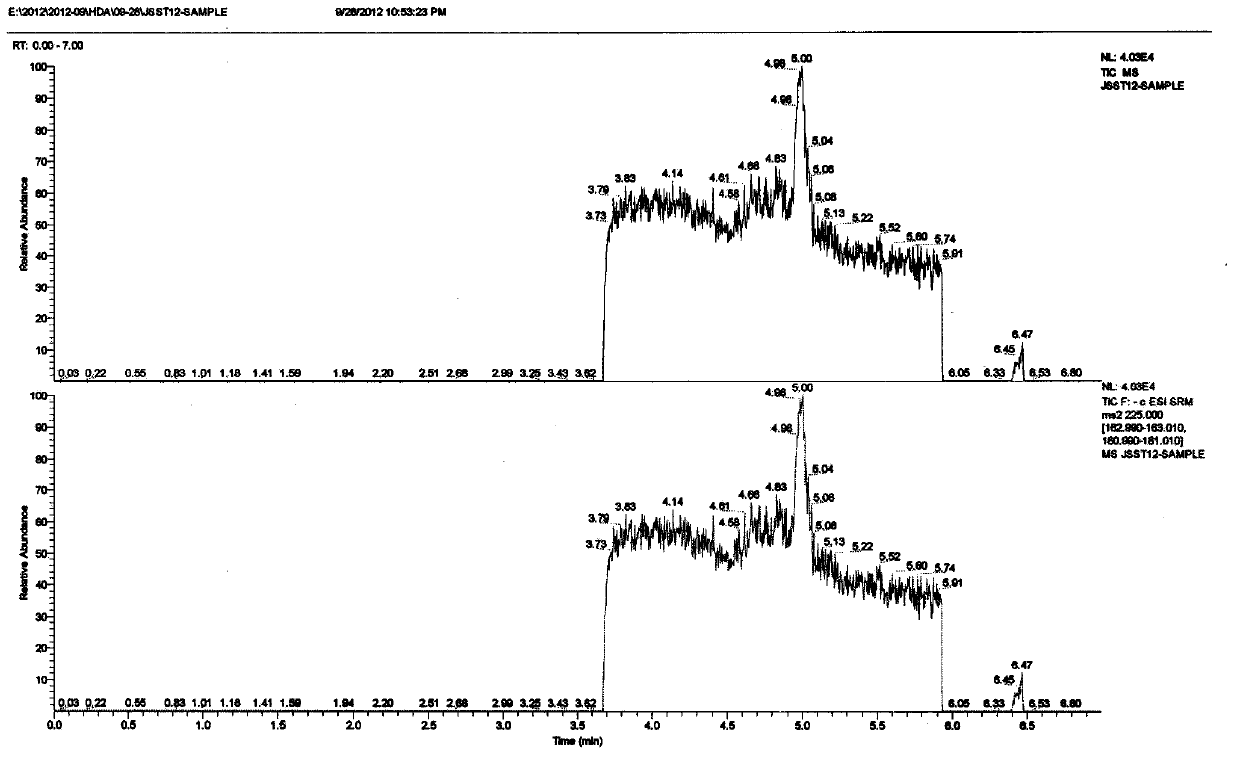
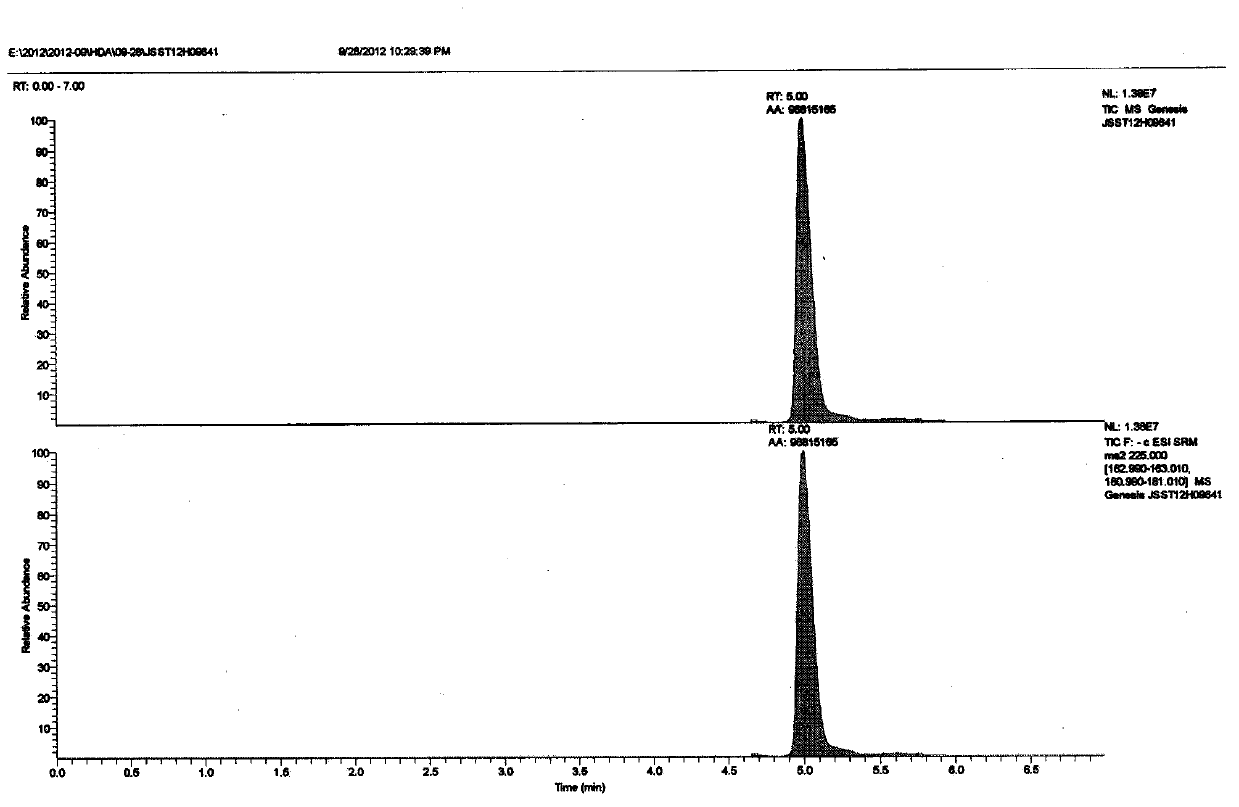
Quick determination method of artificial decenoic acid
The invention belongs to the technical field of food detection, in particular relates to a quick and accurate method for detecting whether pure natural royal jelly and freeze-dried powder are mixed with artificial decenoic acid, and aims to develop a quick and accurate method for qualitative and quantitative detection of the artificial decenoic acid in the royal jelly and the freeze-dried powder in allusion to the foul means of mixing of the artificial decenoic acid in the royal jelly and the freeze-dried powder for seeking high profits in the market in the prior art. The invention discloses the quick determination method of the artificial decenoic acid in the royal jelly and the freeze-dried powder so as to achieve the purpose, and the quick determination method comprises three steps of pretreatment, liquid chromatographic separation and mass spectrometric detection. According to the HPLC-MS detection method, ion peaks with the mass to charge ratio of 225 m / z (parent ion), 163 m / z (daughter ion) and 181 m / z (daughter ion) are taken as feature detection peaks.
Owner:JIANGSU SINOGRAPHY TESTING
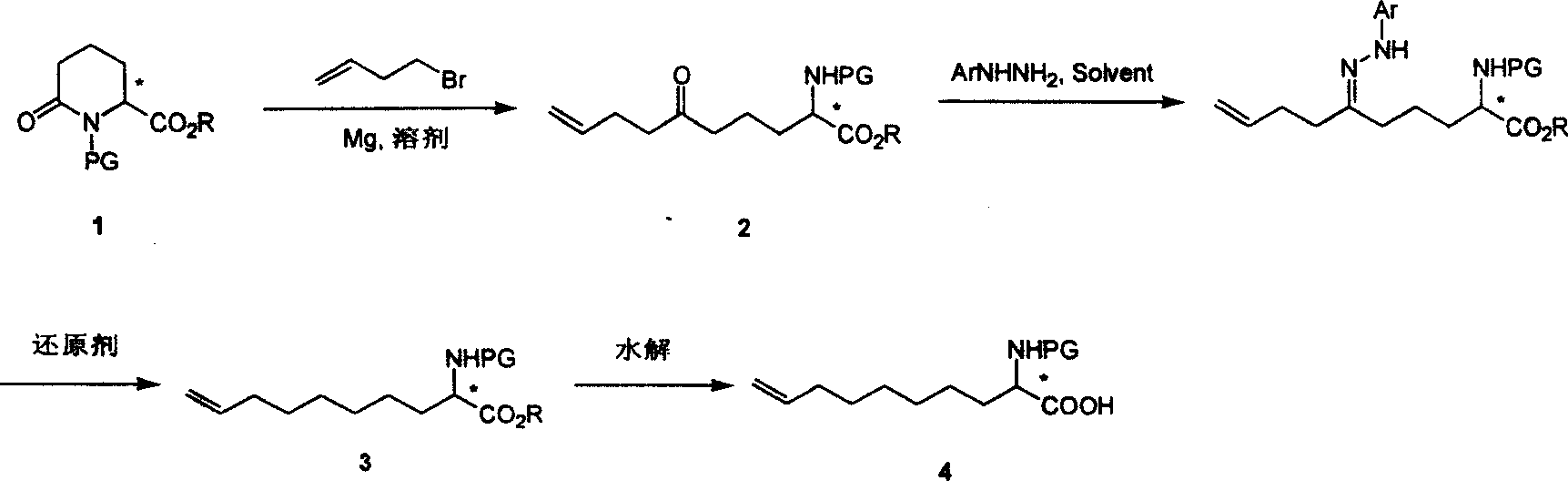
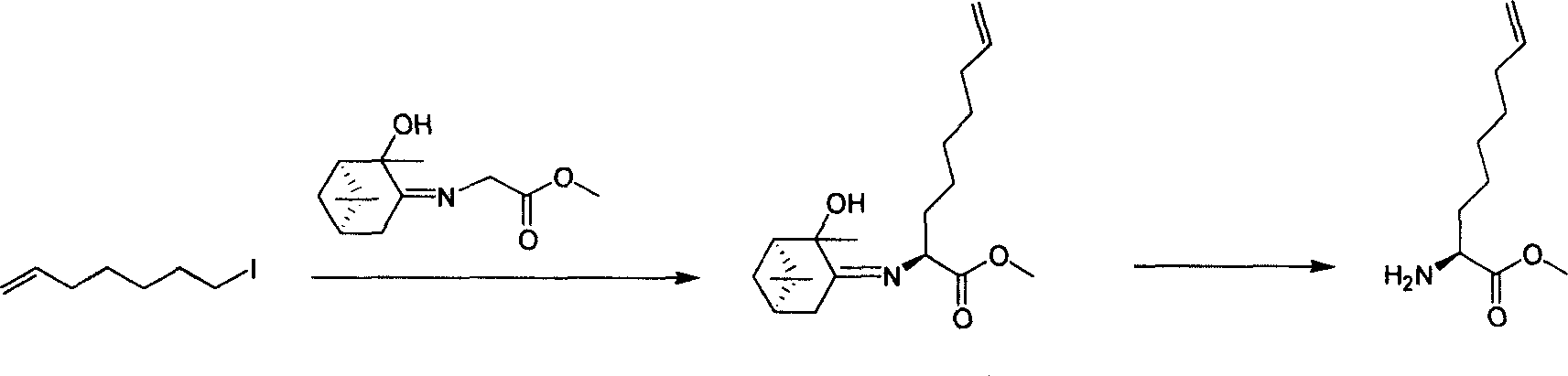
Method for synthesizing 2 - amido - 9 - capric olefine acid
InactiveCN101092368AAvoid expensiveProcess selection is reasonableOrganic compound preparationAmino-carboxyl compound preparationDecenoic AcidPresent method
This invention relates to a method for preparing optically active 2-amino-9-decenoic acid. The method comprises: attacking chiral 6-oxy-piperidine-2-carboxylate with Grignard reagent of crotyl bromide to obtain chiral 2-amino-6-oxy-9-decadienoate, reducing to convert carbonyl into methylene and obtain chiral or racemized 2-amino-9-decadienoate, and hydrolyzing to obtain optically active 2-amino-9-decenoic acid. The method overcomes the problems of long synthesis route, low yield and expensive chiral catalyst or resolution reagent faced by the present method. The method in this invention has such advantages as simple route, reasonable process, low cost, and high yield, and is suitable for mass production.
Owner:上海药明康德新药开发有限公司
Application of E-10-hydroxy-2-decenoic acid in medicines or health products for preventing and treating chemical liver injuries
ActiveCN104622861AImprove antioxidant capacityStrong free radical scavengerOrganic active ingredientsDigestive systemDecenoic AcidSide effect
The invention discloses a novel application of E-10-hydroxy-2-decenoic acid, specifically discloses an applicationof E-10-hydroxy-2-decenoic acid in medicines or health products for preventing and treating chemical liver injuries, and belongs to the technical field of prevention and treatment of chemical liver injuries. E-10-hydroxy-2-decenoic acid has a specific liver protecting effect, can be applied to medicines or health products for preventing and treating the chemical liver injuries, and has the advantages of low cost, good effect, low dosage and no toxic or side effect.
Owner:SHIJIAZHUANG KANGNUO BIOTECH
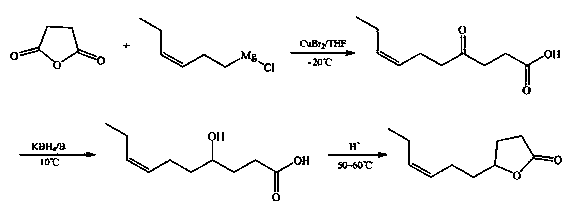
Gamma-3-hexenyl-gamma-butyrolactone synthesis method
InactiveCN103408516AWide variety of sourcesLow priceOrganic chemistryDecenoic AcidPotassium borohydride
The present invention discloses a gamma-3-hexenyl-gamma-butyrolactone synthesis method, which comprises: adopting copper bromide or copper iodide as a catalyst, adopting tetrahydrofuran as a solvent, and carrying out a grignard reaction of succinic anhydride and 1-chloro-cis3-hexene magnesium to obtain an intermediate 4-oxo-7-decenoic acid; under a basic condition, adopting potassium borohydride to reduce the obtained intermediate 4-oxo-7-decenoic acid into 4-hydroxy-7-decenoic acid; and under an acid condition, carrying out a lactonization reaction on the 4-hydroxy-7-decenoic acid to obtain gamma-3-hexenyl-gamma-butyrolactone. The synthesis method has characteristics of wide raw material source, low production cost, high yield, high product purity, simple preparation method and easy operation, and is suitable for industrial production, wherein the yield of the final product gamma-3-hexenyl-gamma-butyrolactone is up to 67.5%, and the purity is up to 99.2%.
Owner:SHANGHAI INST OF TECH +1
Preparation method and application of functional material for adsorption of 10-hydroxy-2-decenoic acid
InactiveCN104829787AHigh selectivityHigh selection specificityComponent separationOther chemical processesDecenoic AcidFunctional monomer
The present invention provides a preparation method and application of a functional material for adsorption of 10-hydroxy-2-decenoic acid. The functional material is synthesized by using 10-hydroxy-2-decenoic acid as a template molecule, 4-vinyl pyridine as a functional monomer, N,N'-methylene bisacrylamide as a crosslinking agent and methanol as a pore forming agent through a sol-gel technology and molecular imprinting technology, and the adsorption capacity of the functional material reaches 22.97mg / g. The synthesis process of the invention is simple, low in cost, and easy to control the reaction conditions; and the prepared specific adsorption material for 10-hydroxy-2-decenoic acid can be used in combination of solid phase extraction enrichment and high performance liquid chromatography, and is applicable to detection of royal jelly and 10-hydroxy-2-decenoic acid in royal jelly products.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
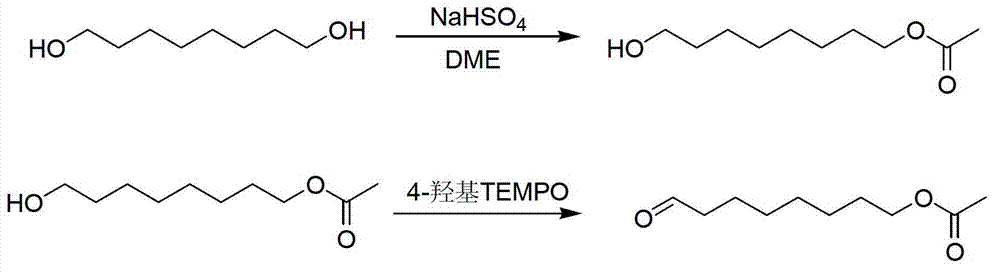
Method for synthesizing 8-acetoxyl octaldehyde
InactiveCN103274933BHigh purityProcess synthesis route simplificationOrganic compound preparationCarboxylic acid esters preparationFood additiveDecenoic Acid
The invention relates to a method for synthesizing 8-acetoxyl octaldehyde, which is an important midbody of a functional food additive 10-hydroxy-2-decenoic acid. The method is characterized in that 1,8-octylene glycol reacts with acetic acid in a dimethoxyethane (DME) solvent under the catalysis of sodium hydrogen sulfate to generate 8-acetoxyl octanol, and oxidizing 8-acetoxyl octanol under the action of 4-hydroxy-2, 2, 6, 6- tetramethyl piperidine nitrogen oxides (4-hydroxy TEMPO) to generate 8-acetoxyl octaldehyde. The method optimizes the existing synthetic route, the design synthesis is carried out in the selective esterification step, and the oxidant 4-hydroxy TEMPO with excellent performance is selected for the oxidation reaction. The method has the advantages that the raw material is economical and easily available, the reaction condition is moderate, the product is easy to purify, the reaction yield is high, environmental friendliness and simplicity in operation can be achieved; and therefore the method is applicable to the industrial production.
Owner:NANJING NORMAL UNIVERSITY
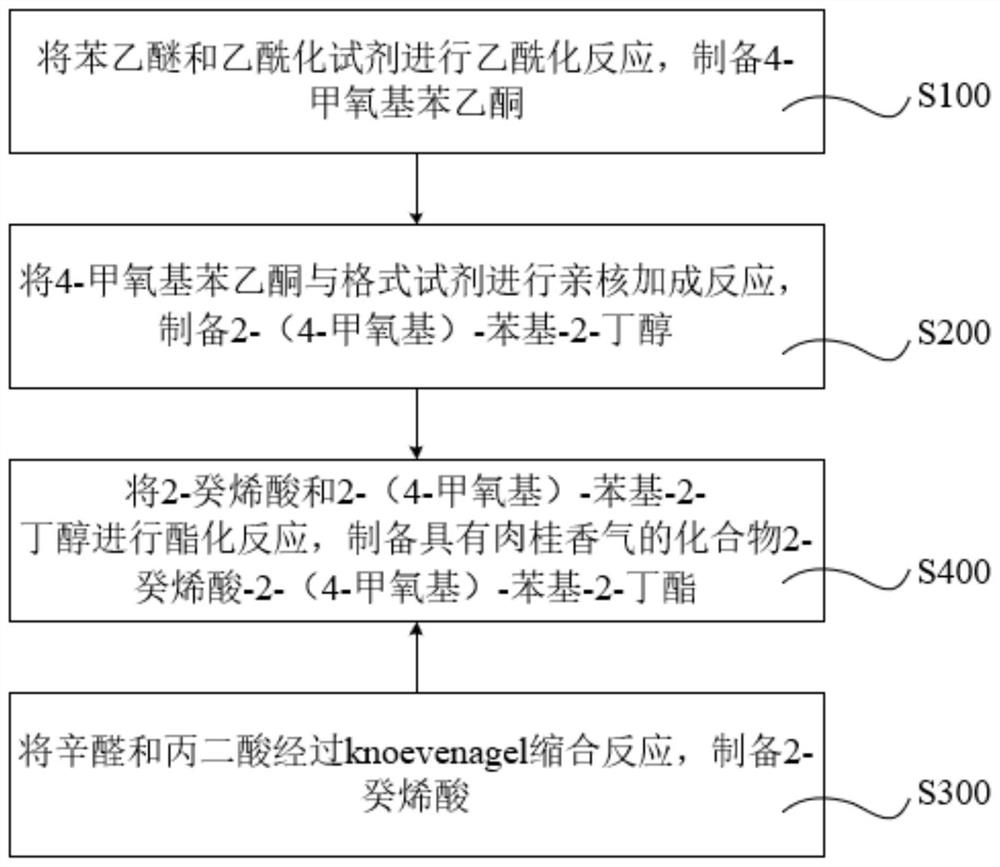
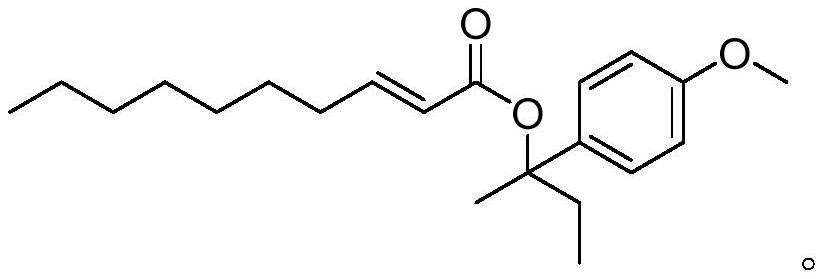
Compound with cinnamon fragrance as well as preparation method and application thereof
ActiveCN112624926AObvious cinnamon aromaStrong and long-lasting cinnamon aromaOrganic compound preparationCarboxylic acid esters preparationDecenoic AcidChinese cinnamon
The invention relates to a compound with cinnamon fragrance as well as a preparation method and application thereof. The compound has the following structural formula, wherein the structural formula can be named as 2-decenoic acid-2-(4-methoxy)-phenyl-2-butyl ester. The provided compound has the advantages of obvious cinnamon aroma, rich aroma, lasting aroma and good stability, and enriches the sources of cinnamon aroma. When the essence is prepared in different periods, the compound has consistent cinnamon aroma, so that the required aroma can be accurately mastered.
Owner:DONGGUAN BOTON FLAVORS & FRAGRANCES
Royal jelly and preparation method thereof
InactiveCN112971079AImprove stabilityReduce the possibility of decompositionFood freezingFood preservationBiotechnologyDecenoic Acid
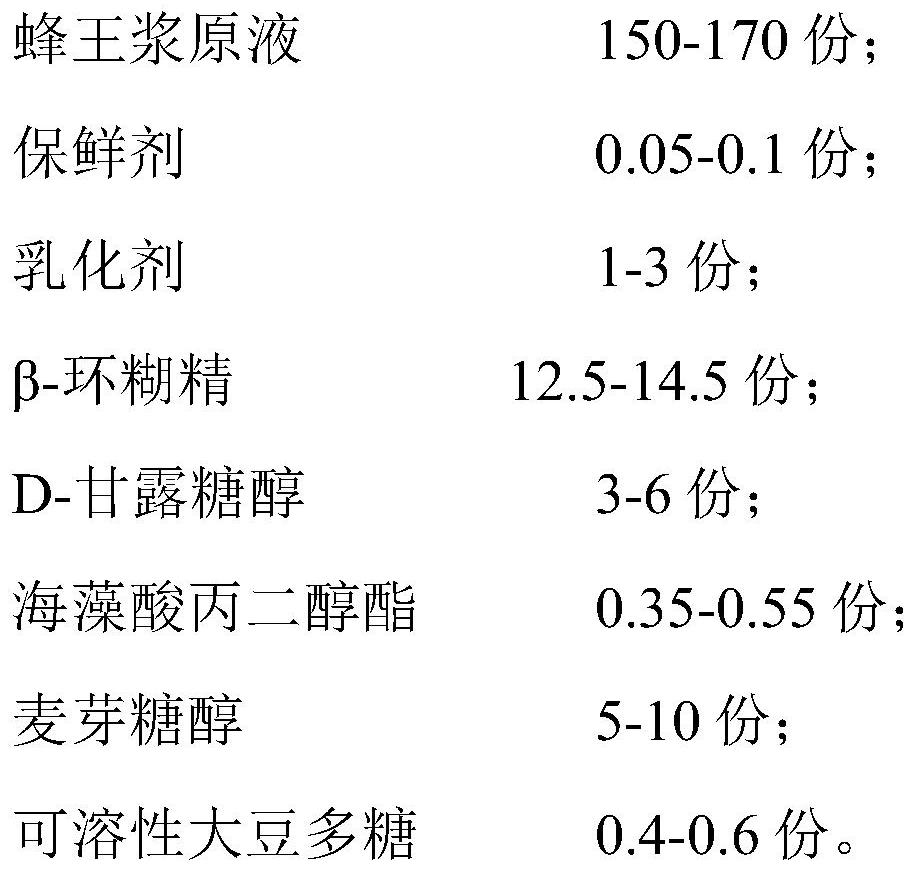
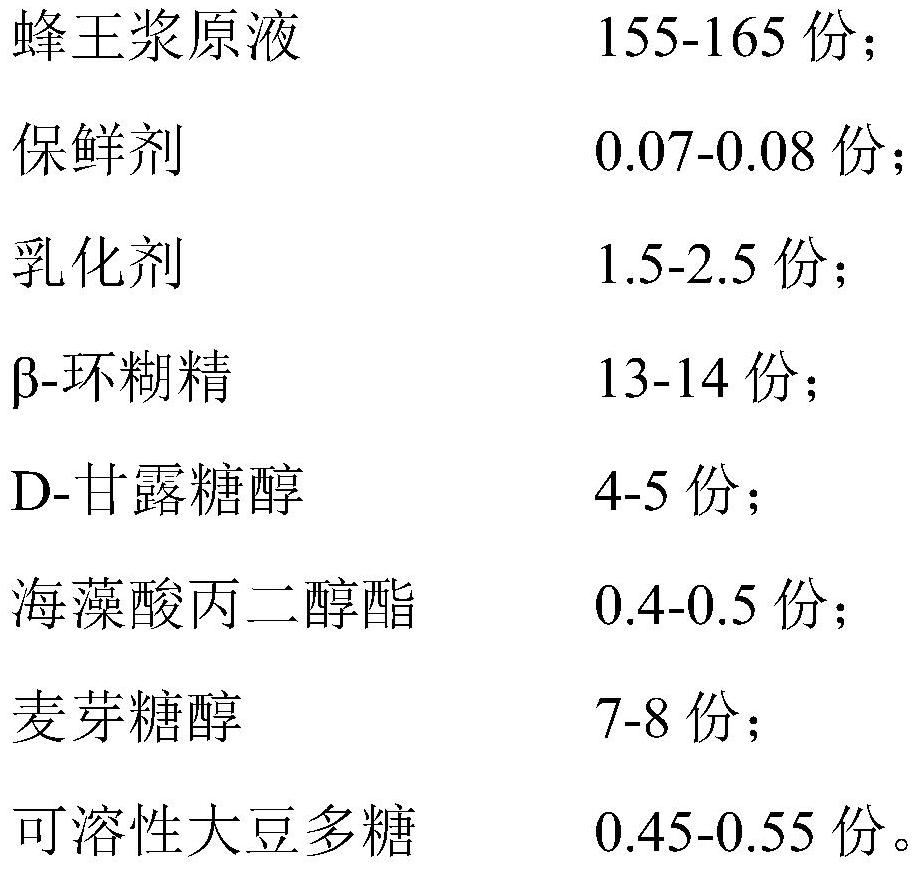
The invention relates to the technical field of royal jelly processing, and particularly discloses royal jelly and a preparation method thereof. The royal jelly is prepared from the following raw material components in parts by weight of 150 to 170 parts of royal jelly stock solution, 0.05 to 0.1 part of preservative, 1 to 3 parts of emulsifier, 12.5 to 14.5 parts of beta-cyclodextrin, 3 to 6 parts of D-mannitol, 0.35 to 0.55 part of propylene glycol alginate, 5 to 10 parts of maltitol and 0.4 to 0.6 part of soluble soybean polysaccharide. The royal jelly disclosed by the invention is relatively high in water-soluble protein stability, relatively low in decomposition rate, relatively high in preservation rate, relatively high in content of an active substance 10-hydroxy-2-decenoic acid, relatively good in taste and relatively long in storage time.
Owner:北京紫云英保健品开发有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com