Cyclic peptide anti-tumor activity compound and preparation method therefor and application of cyclic peptide anti-tumor activity compound
A technology with anti-tumor activity and cyclic peptides, which is applied in the field of medicine, can solve the problems of weak anti-hydrolytic enzyme ability, unstable conformation, and poor membrane penetration ability, so as to improve structural rigidity, enhance cell permeability, and improve enzyme stability. and antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
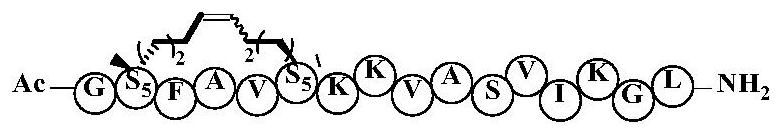
[0042] Example 1: The preparation method of cyclic peptide anti-tumor active compound, solid-phase synthesis of A4K14-Citropin1.1-Sp1, the specific steps are as follows:
[0043]The amino acid α-amino group is protected with 9-fluorenylmethoxycarbonyl (Fmoc), and the side chain of the amino acid is protected: the side chain protection group of Ser is tert-butyl (tBu), and the side chain protection group of Lys is tert-butoxycarbonyl (Boc), wherein the amino acids at the second and fifth positions are replaced with Fmoc-S5-OH, and 6-chlorobenzotriazole-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU ), N,N-diisopropylethylamine (DIPEA) as the activation reagent, the above-mentioned protected amino acids were coupled sequentially, each coupling was 40 minutes, and 20% piperidine / DMF was used as the de-Fmoc reagent, each time was 10 minutes , after the polypeptide is connected, phenylmethylene bis(triphenylhexylphosphine) ruthenium dichloride (the first generation Grubbs cat...
Embodiment 2
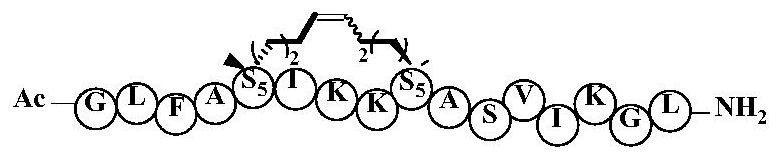
[0044] Example 2: The preparation method of cyclic peptide anti-tumor active compound, solid-phase synthesis of A4K14-Citropin1.1-Sp6, the specific steps are as follows:
[0045] The amino acid α-amino group is protected with 9-fluorenylmethoxycarbonyl (Fmoc), and the side chain of the amino acid is protected: the side chain protection group of Ser is tert-butyl (tBu), and the side chain protection group of Lys is tert-butoxycarbonyl (Boc), wherein the second position is replaced by Fmoc-R8-OH, the ninth amino acid is replaced by Fmoc-S5-OH, and 6-chlorobenzotriazole-1,1,3,3-tetramethylurea Hexafluorophosphate (HCTU) and N,N-diisopropylethylamine (DIPEA) were used as activating reagents, and the above-mentioned protected amino acids were coupled sequentially, each coupling was 40 minutes, and 20% piperidine / DMF was used as de-Fmoc Reagents, 10 minutes each time, after the peptide is connected, phenylmethylene bis(triphenylhexylphosphine) ruthenium dichloride (first-generation ...
experiment example
[0047] 1) Cell Biology Experiments
[0048] CCK-8 tumor suppression experiment in vitro: Prostate cancer cell C42B was cultured in high-glucose D-MEM containing fetal bovine serum (10%), penicillin (100KU·L-1) and streptomycin (100mg·L-1) based on 37 C42B cells in the logarithmic growth phase were seeded in a 96-well plate at a density of 2 × 104mL-1 in a 5% CO2 incubator, with 100μL per well, and 3 replicate wells for each group. The peptide concentration was 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50 μM concentrations act on the cells. After the cells are cultured for 96 hours, add 100 μL of complete medium containing 10% CCK-8 reagent to each well, and incubate at 37°C in a 5% CO2 incubator in the dark After 2h, use a microplate reader (BioTek, Vermont, USA) to detect the absorbance value (OD) of each well at a wavelength of 450nm, and calculate the cell viability (vitalrate, VR) according to the OD value: VR=(OD value of drug group-blank group OD value) / (control group OD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com