Cyclopeptide antitumor active compound and preparation method and application thereof
An anti-tumor activity, cyclic peptide technology, applied in the field of medicine, can solve the problems of poor membrane permeability, unstable conformation of anti-cancer drugs, weak anti-hydrolase ability, etc., to improve anti-tumor activity, improve enzyme stability and Antitumor activity, the effect of enhancing cell permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
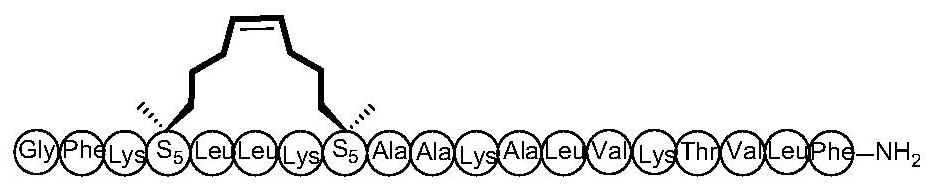
[0039] Example 1: The preparation method of cyclic peptide anti-tumor active compound, solid-phase synthesis of Ascaphin-8-1, the specific steps are as follows:
[0040] The amino resin protected by 9-fluorenylmethoxycarbonyl (Fmoc) is swelled, and the Fmoc must be removed before coupling. Replace the fourth and eighth amino acids with Fmoc-S5-OH, with 6-chlorobenzotriazole-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU), N,N - Diisopropylethylamine (DIPEA) is used as an activating reagent, and the above-mentioned protected amino acids are sequentially coupled, and each coupling takes 40 minutes. After each coupling of an amino acid, 20% piperidine / DMF should be used as the de-Fmoc reagent to de-Fmoc, 10 minutes each time. After the peptides were linked, phenylmethylene bis(triphenylhexylphosphine) ruthenium dichloride (first-generation Grubbs catalyst) was used as a cyclization reagent and reacted overnight. Use TFA / EDT / TIPs / Water (95:2:2:1, v / v / v / v) for 2 hours at ro...
Embodiment 2
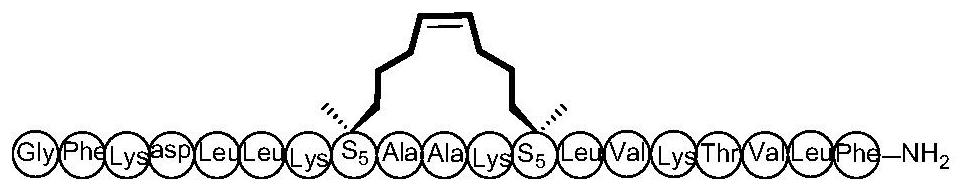
[0041] Example 2: The preparation method of cyclic peptide anti-tumor active compound, solid-phase synthesis of Ascaphin-8-4, the specific steps are as follows:
[0042]The amino resin protected by 9-fluorenylmethoxycarbonyl (Fmoc) is swelled, and the Fmoc must be removed before coupling. The ninth amino acid was replaced with Fmoc-R8-OH, and the seventeenth amino acid was replaced with Fmoc-S5-OH. Using 6-chlorobenzotriazole-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU) and N,N-diisopropylethylamine (DIPEA) as activating reagents, the above protection Amino acids were coupled sequentially, 40 minutes per coupling. After each coupling of an amino acid, 20% piperidine / DMF should be used as the de-Fmoc reagent to de-Fmoc, 10 minutes each time. After the peptides were linked, phenylmethylene bis(triphenylhexylphosphine) ruthenium dichloride (first-generation Grubbs catalyst) was used as a cyclization reagent and reacted overnight. Use TFA / EDT / TIPs / Water (95:2:2:1, v / v / ...
experiment example
[0044] 1) Cell Biology Experiments
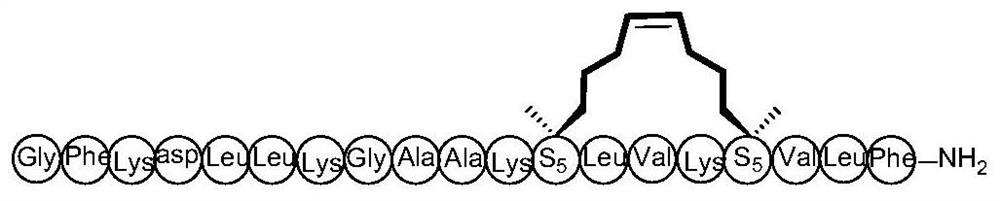
[0045] Prostate cancer bone metastasis cell line C4-2B was cultured in high glucose D-MEM containing 10% fetal bovine serum, 100 U / ml penicillin and 100 mg·L-1 streptomycin at 37°C, 5% CO2 Routine culture and passage in the incubator. Prostate cancer bone metastases cell line C4-2B was plated in a 96-well plate with 1000 cells per well, and peptides Ascaphin-8 and Ascaphin-8 of different concentrations (0, 3.125, 25, 12.5, 25, 50, 100um) were added the next day -1, Ascaphin-8-2, Ascaphin-8-3, Ascaphin-8-4. After 96 hours, add 10 μL of CCK8 reagent to each well and incubate at 37°C for 1 hour. Use a microplate reader (BioTek, Vermont, USA) to detect the absorbance value (OD) of each well at a wavelength of 450 nm, and calculate the cell viability (vital rate, VR) according to the OD value: VR=(OD value of the drug group-OD value of the blank group ) / (OD value of control group-OD value of blank group). Calculate the average VR of 3 paralle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com