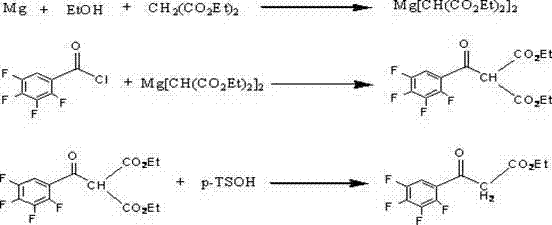
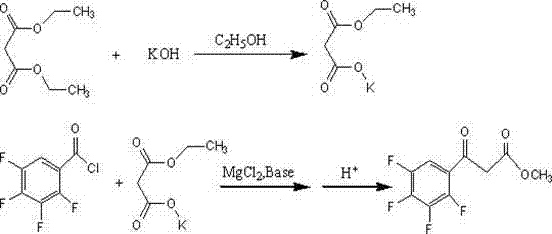
Preparation method of ethyl (2,3,4,5-tetrafluorobenzoyl) acetate
A technology of ethyl tetrafluorobenzoyl acetate and tetrafluorobenzoyl chloride is applied in the field of preparation of pharmaceutical intermediate 2,3,4,5-ethyl tetrafluorobenzoyl acetate, and can solve harsh conditions and overall The problems of low yield and high risk can achieve the effect of saving energy and overcoming restrictions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. In a 500mL round-bottomed flask equipped with a thermometer, mechanical stirring, and a reflux condenser, add 125mL of absolute ethanol and 35 (0.22mol) g of monoethyl malonate, stir and dissolve at room temperature, and weigh 12.3g in a beaker (0.22mol) Potassium Hydroxide, dissolved in 125mL absolute ethanol to make a solution. Keep the temperature in the bottle at 20-25°C, start to drop the ethanol solution of potassium hydroxide, control the temperature in the bottle at 20-25°C, add dropwise for about 1 hour, then stir and react at this temperature for 12 hours, the reaction system Heat up to about 80°C to dissolve, stir and reflux for 1 hour, cool down to below -5°C, a flaky solid precipitates in the bottle, wash with a small amount of ether after suction filtration, and dry to obtain 29.5g of white flaky potassium monoethyl malonate (theoretical yield 95%), the next reaction can be carried out directly without purification.
[0020] 2. In a 500mL four-neck ro...
Embodiment 2
[0022] 1, the preparation of monoethyl malonate potassium is with example 1.
[0023] 2. In a 500mL four-neck round bottom flask, add 23.8g (0.14mol) monoethyl malonate potassium salt, 190.4g dichloromethane, stir and mix, cool the solution to 0°C, and add 35.4g triethylamine (0.35mol), 6g (0.168mol) of anhydrous magnesium chloride, and then 21.2g (0.1mol) of 2,3,4,5-tetrafluorobenzoyl chloride was added dropwise, and the addition was completed in about half an hour. The temperature was raised to 25°C, and the reaction was stirred for 10 hours. After the reaction of the raw materials was detected by TLC, the organic phase was separated, washed twice with 50 mL of 13% hydrochloric acid, and washed twice with 50 mL of water. Dry the organic phase with anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, add 20g of petroleum ether to dissolve, then cool down to 0°C to precipitate a white flaky solid, and filter it to obtain the product 2,3,4,5-tetrafluoroben...
example 3
[0025] 1, the preparation of monoethyl malonate potassium is with example 1.
[0026] 2. In a 500mL four-neck round bottom flask, add 23.8g (0.14mol) monoethyl malonate potassium salt, 142.8 g dichloromethane, stir and mix, cool the solution to 0°C, and add 35.4g triethylamine (0.35mol), 6g (0.168mol) of anhydrous magnesium chloride, and then 21.2g (0.1mol) of 2,3,4,5-tetrafluorobenzoyl chloride was added dropwise, and the addition was completed in about half an hour. Raise the temperature to 20°C, stir and react for 15 hours. After the reaction of the raw materials was detected by TLC, the organic phase was separated and washed twice with 50 mL of 13% hydrochloric acid each time, and then washed twice with 50 mL of water. Dry the organic phase with anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, add 20g of petroleum ether to dissolve, then cool down to 0°C to precipitate a white flaky solid, and filter it to obtain the product 2,3,4,5-tetrafluoroben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com