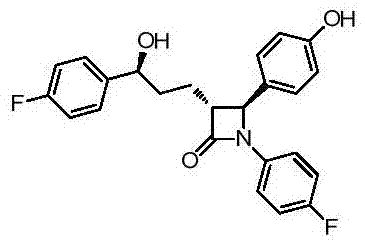
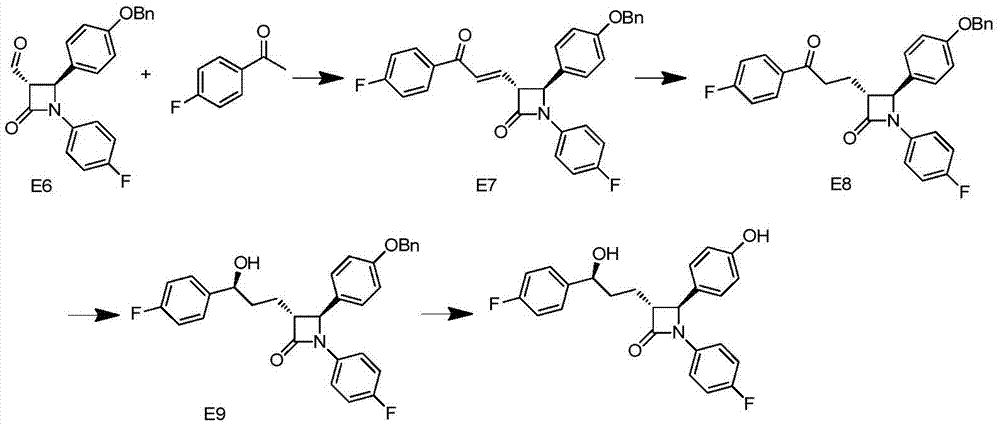
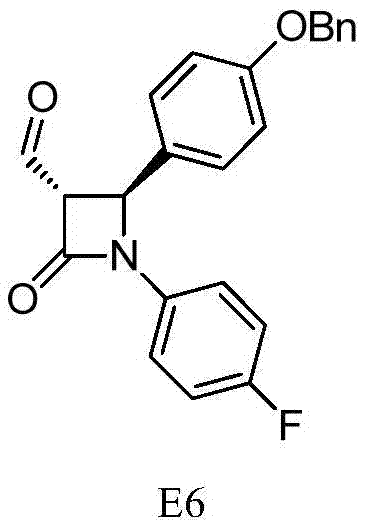
Synthesis process of ezetimibe intermediate
A synthetic process and intermediate technology, which is applied in the field of synthetic process of ezetimibe intermediates, can solve the problems of lack of actual combat experience and synthetic routes that cannot reach large-scale production, and achieve high total yield, low production cost, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 intermediate E3
[0034] (S)-3-oxo-3-(2-oxo-4-phenyloxazolin-3-yl) ethyl propionate, the structural formula is as follows:
[0035]
[0036] In a 500ml three-necked flask, add (s)-4-phenyl-2-oxazolone 20g (0.122mol, 1eq.) and dichloromethane 200ml, cool to between 0 and 10°C, add TMSCl raw material 16g (0.146 mol, 1.2eq.), keep stirring at 0~10℃ for 30 minutes, add 15.4g (0.152mol, 1.25eq.) of triethylamine dropwise, control the material temperature between 0~10℃ during the dropping process, add After completion, continue to stir at 0-10° C. for 2 hours until the reaction of ((s)-4-phenyl-2-oxazolone is detected by TLC (TLC detection condition: petroleum ether / ethyl acetate=2 / 1). Then add 36.8g monoethyl malonate acid chloride (0.245mol, 2eq.), the dropwise addition process controls the temperature of the material between 0~10°C, after the dropwise addition is completed, add 0.2g anhydrous tetrabutylammonium fluoride ( TBAF, 0.6mmol,...
Embodiment 2
[0037] The preparation of embodiment 2 intermediate E4
[0038] 3-(4-(Benzyloxy)phenyl)-3-((4-fluorophenyl)amino)-2-((S)-2-oxo-4-phenyloxazoline-3-carbonyl) Ethyl propionate, the structural formula is as follows:
[0039]
[0040]In a 250ml three-necked flask, 2.5g of the compound (S)-3-oxo-3-(2-oxo-4-phenyloxazolin-3-yl) ethyl propionate (9mmol, 1eq), 3.3g of compound N-(4-fluorophenyl)-4-benzyloxybenzylideneamine (11mmol, 1.2eq) and 100ml of dichloromethane, cooled to -30°C under nitrogen protection, added 1.75g Diisopropylethylamine (13mmol, 1.5eq), after stirring for 10 minutes, add 2.1g of titanium tetrachloride (11mmol, 1.2eq), the reaction solution is reddish brown, continue stirring at -30~-20°C for 3 hours , after the completion of the reaction, add 0.5ml acetic acid to quench the reaction, add 10% aqueous sodium bisulfite solution of 50ml, stir at room temperature for 1 hour, separate the organic phase, and then wash the organic phase twice with 100ml water to o...
Embodiment 3
[0041] The preparation of embodiment 3 intermediate E5
[0042] (2S,3R)-2-(4-(Benzyloxy)phenyl)-1-(4-fluorophenyl)-4-oxazetidinone-3-ethyl carbonate, the structural formula is as follows:
[0043]
[0044] In a 250ml three-necked flask, add 30g of the compound 3-(4-(benzyloxy)phenyl)-3-((4-fluorophenyl)amino)-2-((S)-2 prepared in Example 2 -Oxy-4-phenyloxazoline-3-carbonyl)propanoic acid ethyl ester (0.05mol, 1eq), 50gBSA (0.24mol, 4.8eq) and 500ml of dichloromethane, heated to 50°C and refluxed for 3 hours, then added 1g of tetrabutylammonium fluoride, continue to keep reflux for 3 hours, after the reaction is finished, cool down to normal temperature, add 500ml water to wash the organic phase twice, separate the organic phase, and remove the solvent by distilling the organic phase under reduced pressure, and then reapply in 200ml toluene Crystallized, separated and dried to obtain product 15g, yield 75%, MSm / z: 420 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com