Synthesis method and device for 4-hydroxyquinoline-3-formic acid
A technology of hydroxyquinoline and synthetic method, which is applied in chemical instruments and methods, chemical/physical/physical chemical processes, organic chemistry, etc., can solve the problems of high cost, troublesome post-processing, long reaction time, etc., and achieve short reaction time , easy post-processing, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] Embodiments of the present invention are described in detail below, examples of which are shown in the drawings, wherein the same or similar reference numerals designate the same or similar elements or elements having the same or similar functions throughout. The embodiments described below by referring to the figures are exemplary and are intended to explain the present invention and should not be construed as limiting the present invention.
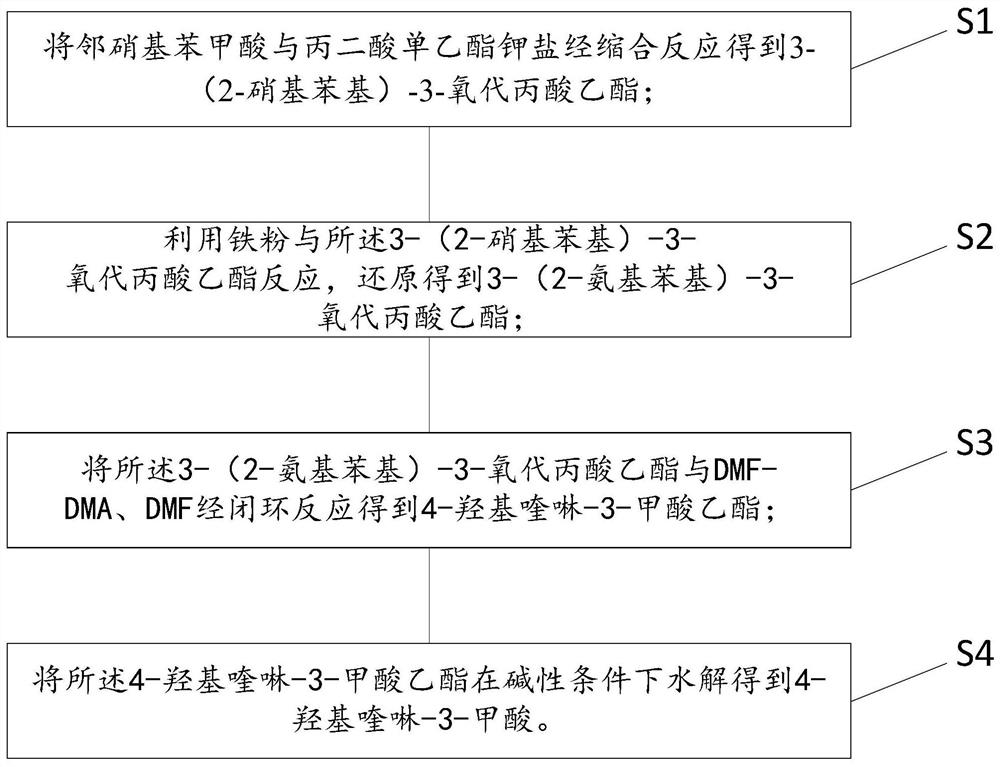
[0054] see Figure 1 to Figure 13 , the invention provides a kind of synthetic method of 4-hydroxyquinoline-3-formic acid, comprises the steps:
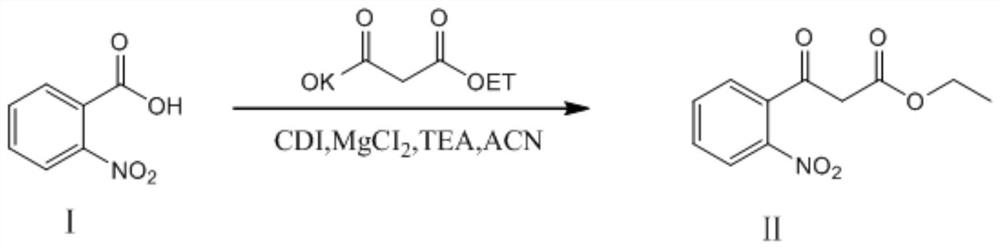
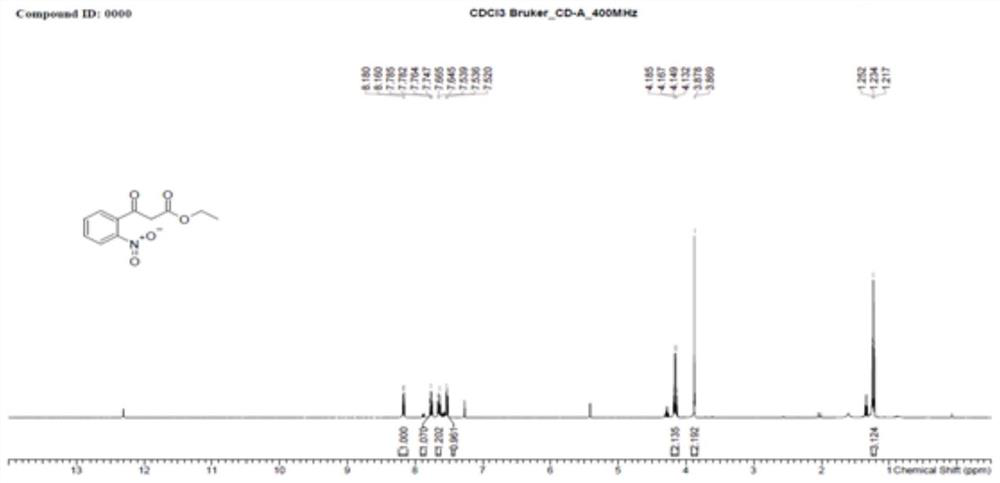
[0055] S1: Condensation reaction of o-nitrobenzoic acid and monoethyl malonate potassium salt to obtain ethyl 3-(2-nitrophenyl)-3-oxopropionate;
[0056] S2: using iron powder to react with the ethyl 3-(2-nitrophenyl)-3-oxopropionate, and reducing it to obtain ethyl 3-(2-aminophenyl)-3-oxopropionate;
[0057] S3: The 3-(2-aminophenyl)-3-oxopropionic acid ethyl ester is reacted with DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com