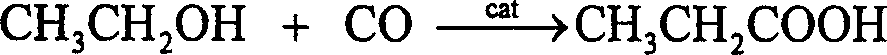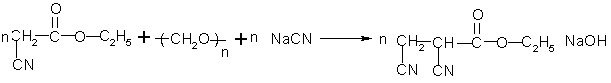Patents
Literature
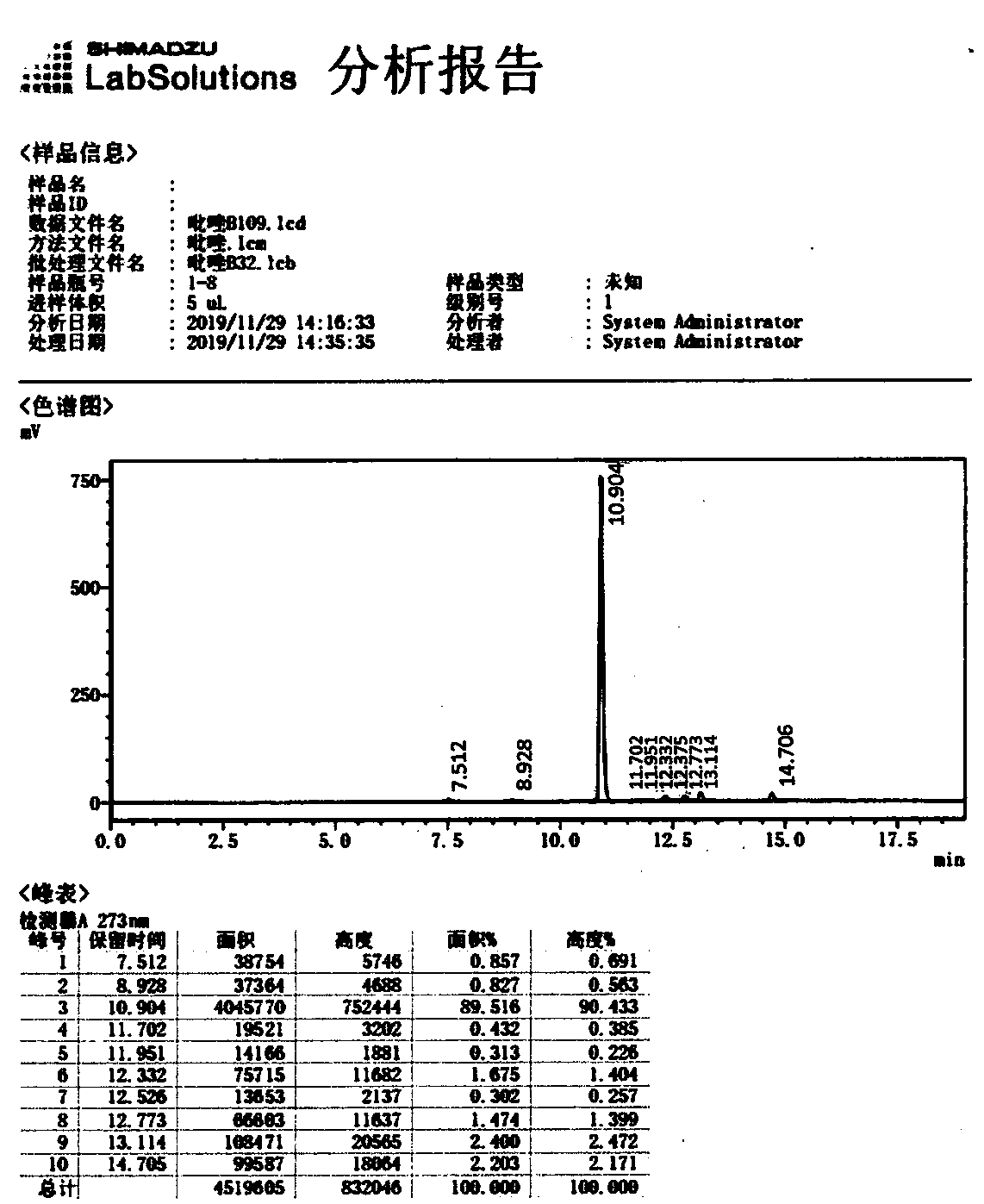
146 results about "Ethyl propiolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
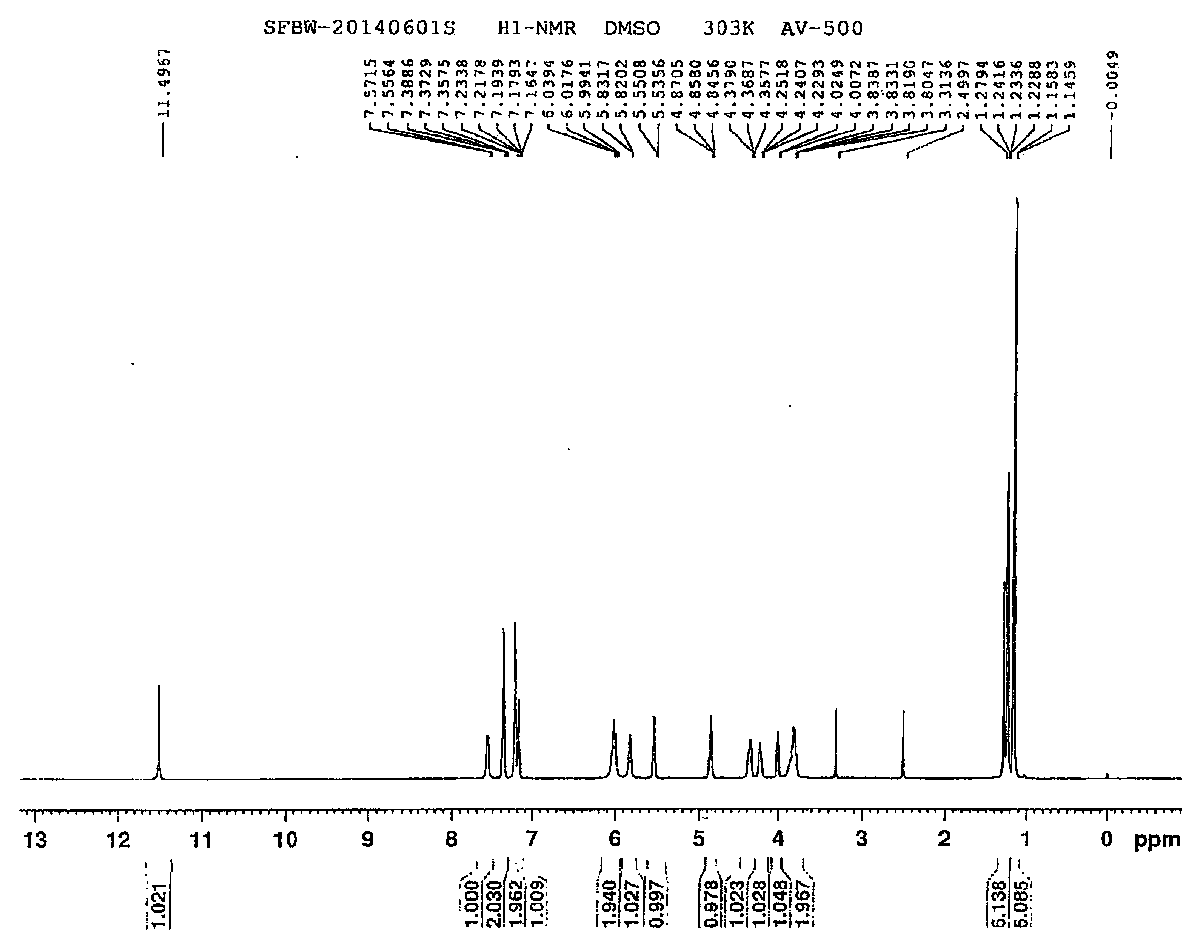
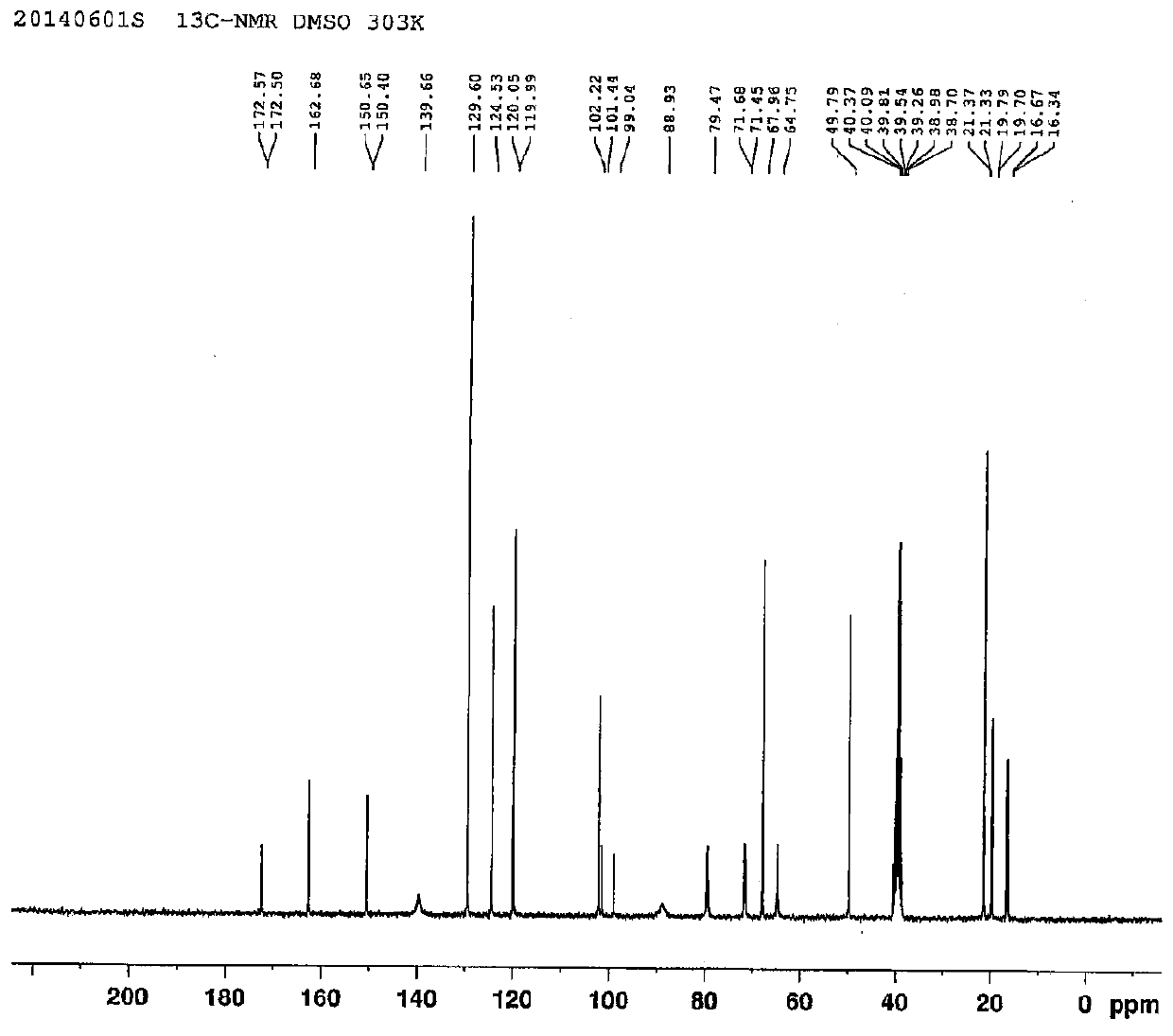
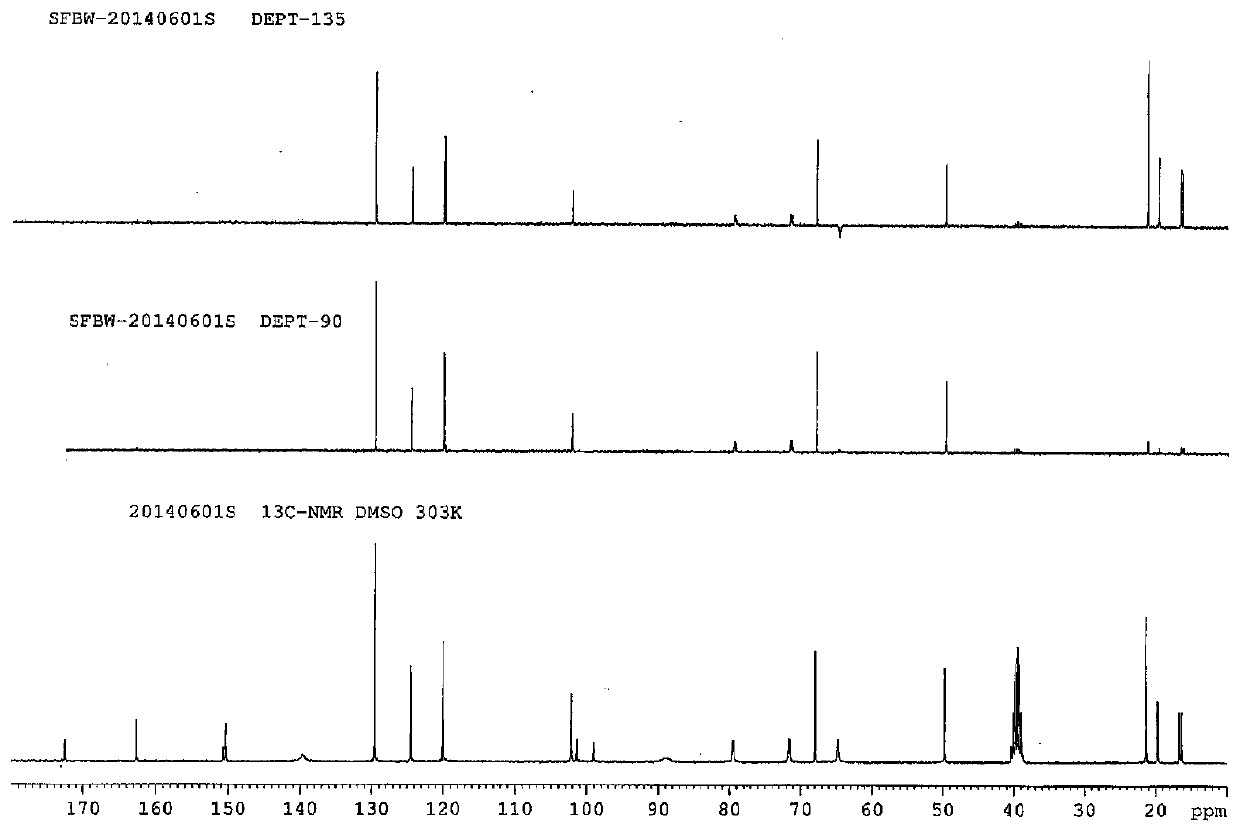
Ethyl propiolate is an organic compound with the formula HC₂CO₂C₂H₅. It is the ethyl ester of propiolic acid, the simplest acetylenic carboxylic acid. It is a colorless liquid that is miscible with organic solvents. The compound is a reagent and building block for the synthesis of other organic compounds, reactions that exploit the electrophilicity of the alkyne group.
Lithium ion battery electrolyte with both high and low temperature performances
The invention relates to the technical field of lithium ion electrolytes, and in particular relates to a lithium-ion battery electrolyte with both high and low temperature performances. The electrolyte comprises lithium hexafluorophosphate, mixed organic solvents, filming additives, additives for improving the dielectric constant and the low temperature infiltration capability, and a lithium salt type additive, wherein the mixed organic solvents comprise a carbonic ester solvent and a linear carboxylic ester solvent; the linear carboxylic ester solvent in the mixed organic solvents is one or a mixture of more than two of ethyl propionate, propionic acid n-propyl ester, n-propyl acetate, acetic acid n-butyl ester and isobutyl acetate; and the additives for improving the dielectric constant and the low temperature infiltration capability are one or a mixture of more than two of fluoro ethylene carbonate, difluoro ethylene carbonate and 4-trifluoromethyl ethylene carbonate. A battery prepared from the lithium-ion battery electrolyte with both high and low temperature performances is long in service life, and both the good low temperature discharge performance of the battery is ensured, and the storage performance of the battery at the high temperature of 60 DEG C is effectively considered.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS
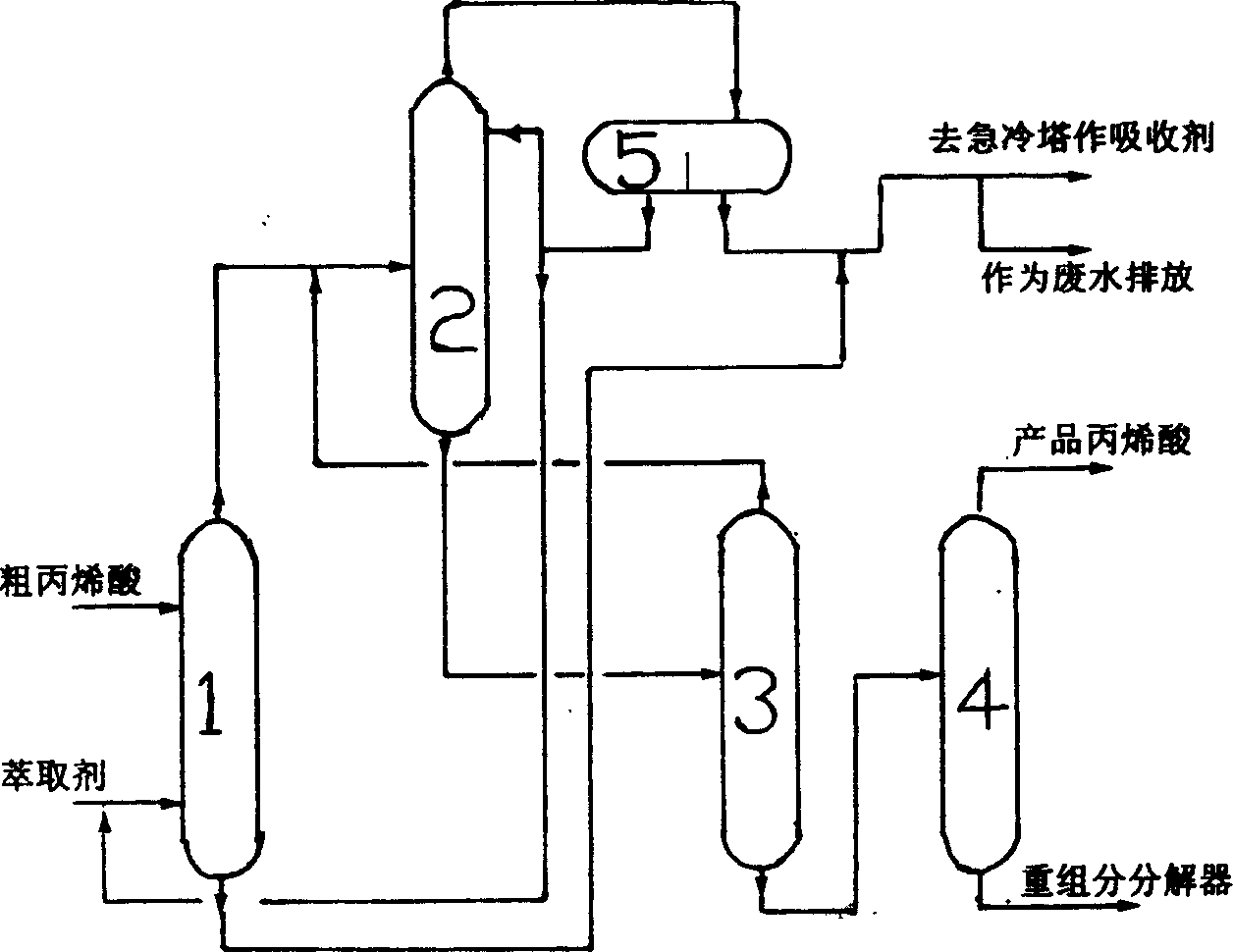
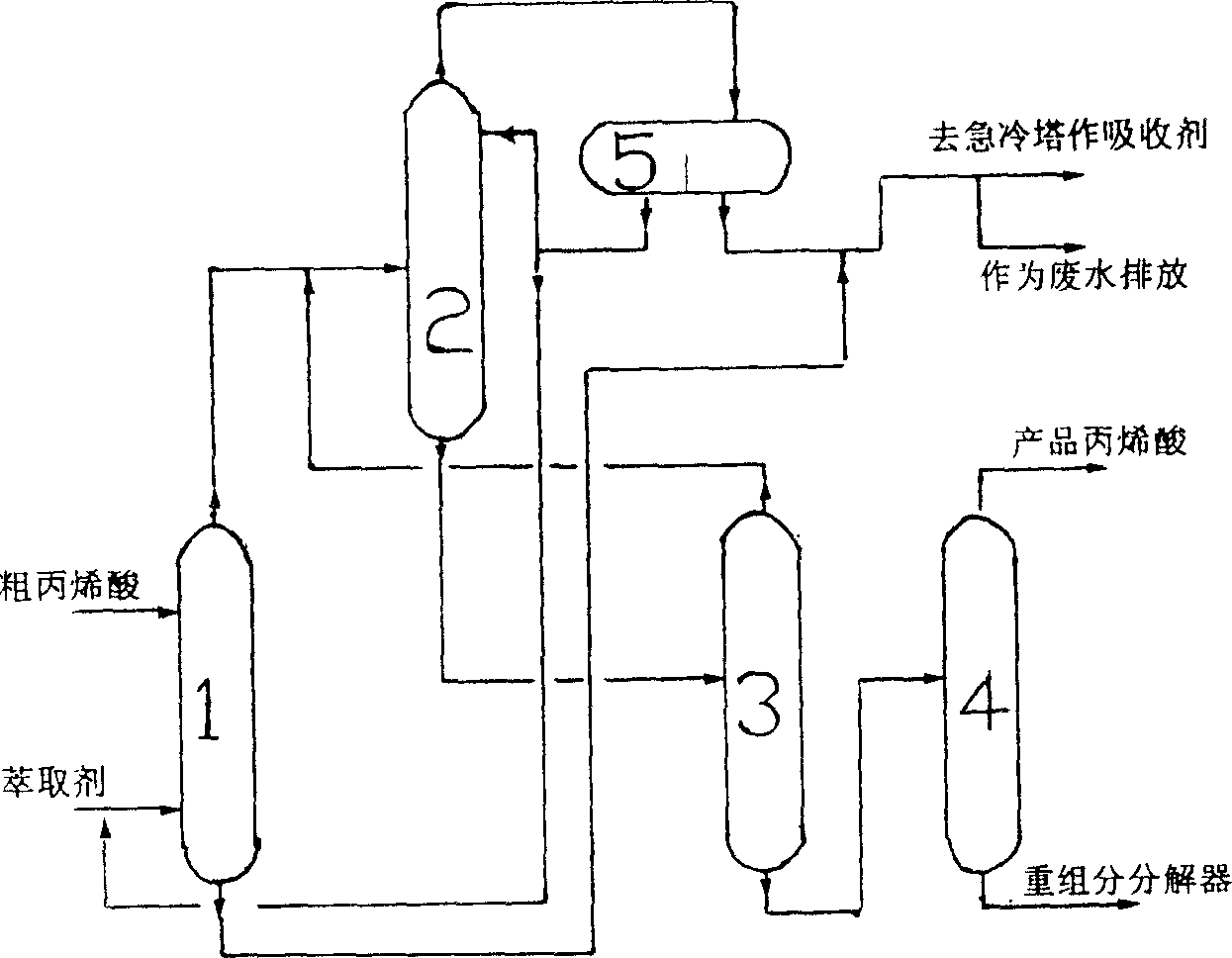
Method for purifying acrylic acid
The invention relates to the process for purifying acrylic acid which comprises using novel organic extracting agent to separate acrylic acid from its water solution, then removing light compositions such as water and acetic acid by azeotropic fractional distillation, wherein the organic extracting agent comprises toluene, acetic ether, butyl acetate, butyl acetate, propionic ether, diisobutyl ketone, isophorone, methyl phenyl ether or their mixture, preferably the composite compound of toluene with isobutyl phenylacetate, the mass ratio of toluene and isobutyl phenylacetate is between 1:1-1:50, preferably 1:2-1:12. The process according to the invention can realize high acetic acid removing ratio.
Owner:SHANGHAI HUAYI NEW MATERIAL
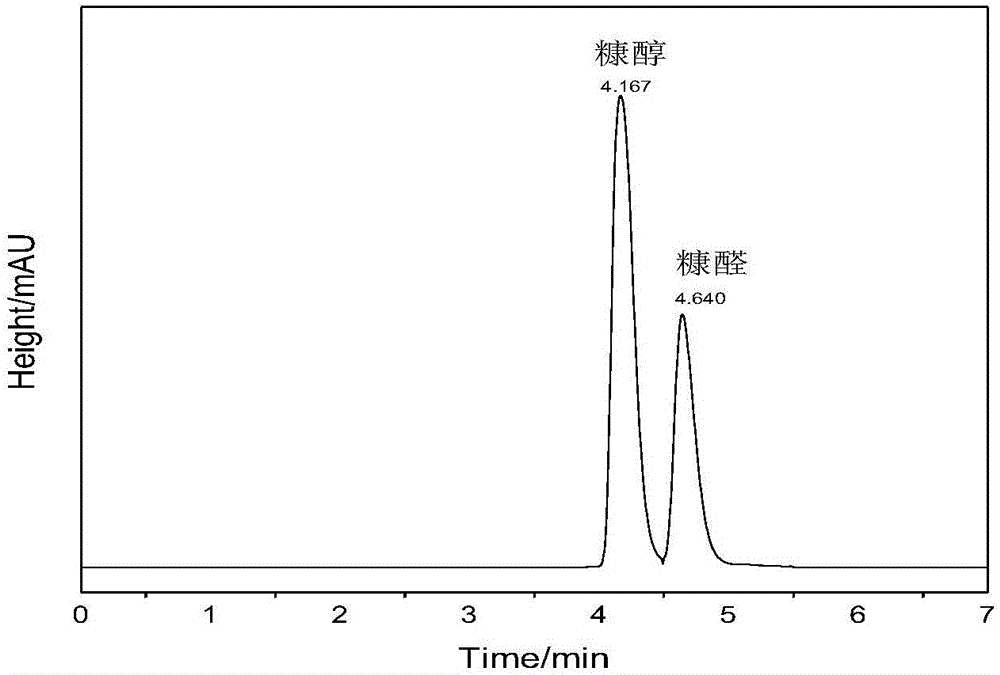
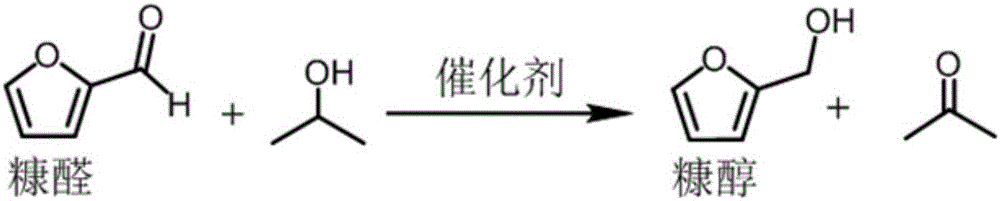
Method for preparing furfuryl alcohol by utilizing hydrogen transfer reaction to catalyze furfural
InactiveCN106543115ASimple processEasy to operateOrganic chemistryPhysical/chemical process catalystsReaction temperatureGamma-Valerolactone
The invention provides a method for preparing furfuryl alcohol by utilizing a hydrogen transfer reaction to catalyze furfural. The method comprises the following steps that under the catalysis of a heterogeneous catalyst, namely magnetic hydroxyapatite, the furfural and alcoholic compounds with hydrogen donors are subjected to the hydrogen transfer reaction, wherein the ratio of the magnetic hydroxyapatite to the alcoholic compounds to the furfural is 20-120 g to 8-20 L to 1 mol. A reaction container is filled with nitrogen (N2) of which pressure is 1-20 bar at room temperature, the reaction temperature is 100 DEG C-200 DEG C, and a reduced product, namely the furfuryl alcohol, is obtained after 1-12 h. The method for preparing the furfuryl alcohol by utilizing the hydrogen transfer reaction to catalyze the furfural is simple in process, convenient to operate and safe and environmentally friendly. The catalyst is a non-precious metal catalyst and cheap and easy to obtain, has magnetism and is easy to separate, and can be reused repeatedly. The activity does not decrease. The industrial cost of preparing the furfuryl alcohol can be lowered to a large extent, ethyl levulinate can be catalyzed to react and gamma-valerolactone is generated, and other compounds containing C=O bonds can be also reduced.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for preparing ethyl levulinate based on solid superacid catalysis and furfuryl alcohol alcoholysis
InactiveCN103274942ALow costEasy to separateOrganic compound preparationCarboxylic acid esters preparationAlcoholEthyl ester
The invention discloses a method for preparing ethyl levulinate based on solid superacid catalysis and furfuryl alcohol alcoholysis, and relates to ethyl levulinate. The method comprises the steps of adding furfuryl alcohol, a catalyst and a reaction solvent to a reaction kettle, reacting, and obtaining ethyl levulinate. The catalyst is selected from at least one of solid superacid of SO4<2-> / TiO2, SO4<2-> / ZrO2, SO4<2-> / Fe2O3, SO4<2-> / SnO2, and SO4<2-> / Al2O3, or at least one of solid superacid of S2O8<2-> / TiO2, S2O8<2-> / ZrO2, S2O8<2-> / Fe2O3, S2O8<2-> / SnO2, and S2O8<2-> / Al2O3; the reaction solvent is ethyl alcohol; and reaction conditions are that the temperature is 100-150 DEG C, the rotating speed is 300-500 rpm (revolutions per minute), the time is 0.5-2.5h, a mass ratio of furfuryl alcohol to solid superacid is (1-4):1, and a mass ratio of furfuryl alcohol to ethyl alcohol is (0.01-0.2):1.
Owner:XIAMEN UNIV
Method for preparing quizalofop-p-ethyl
InactiveCN101333194AAvoid it happening againAlleviate environmental pressureOrganic chemistryQuinoxalineEthyl propiolate
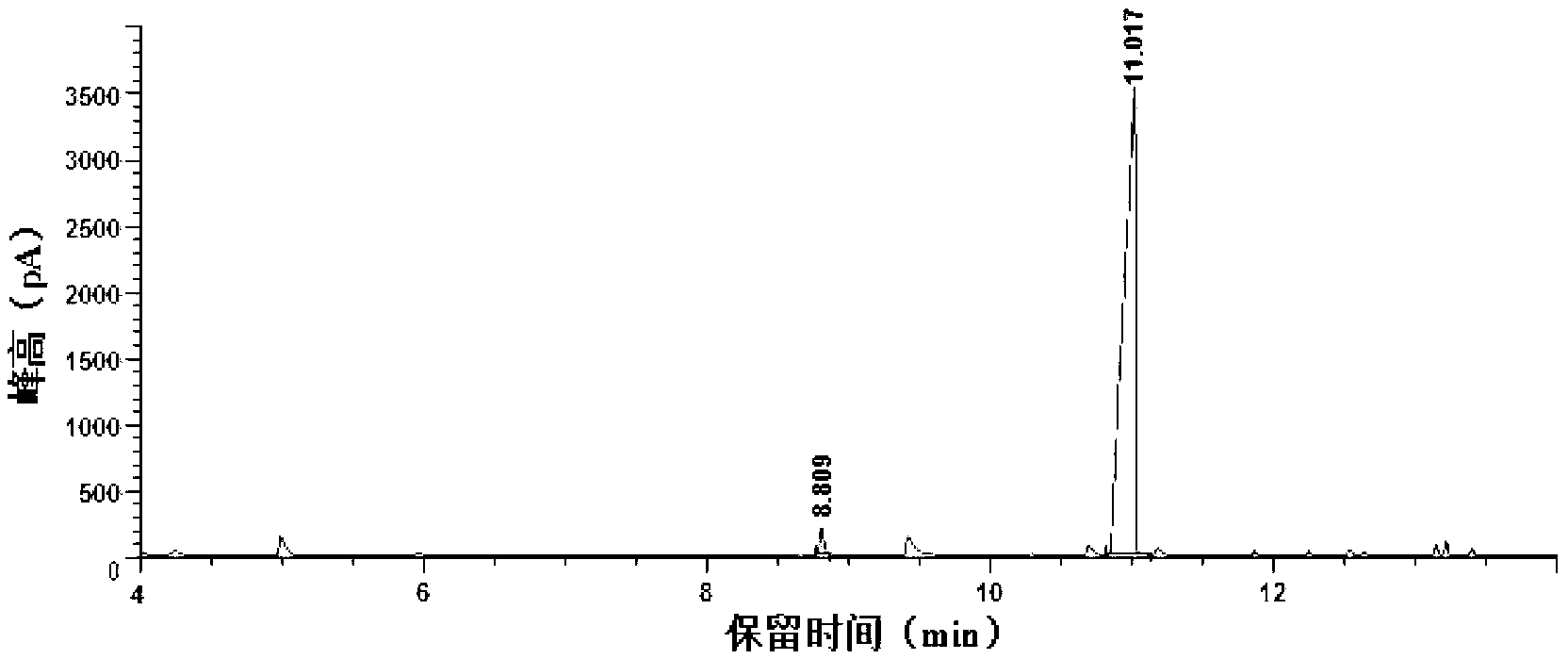
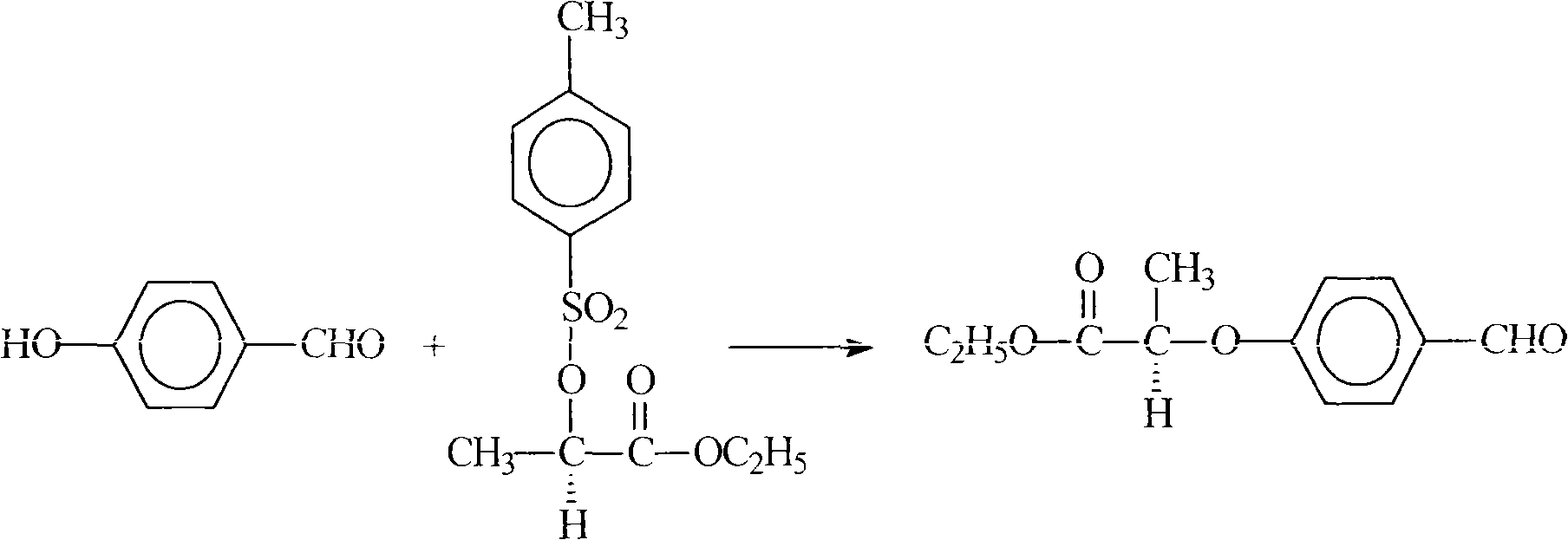
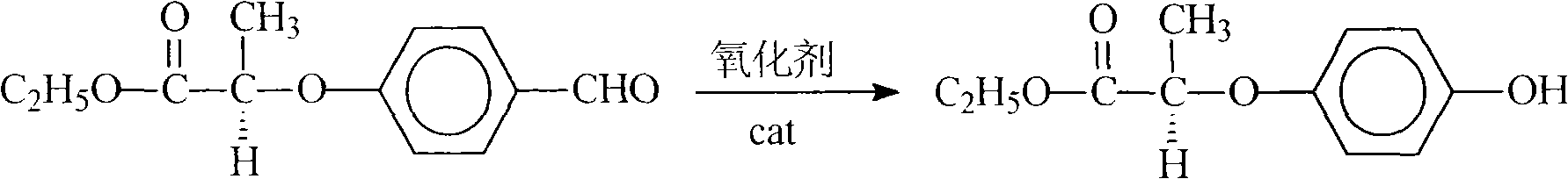
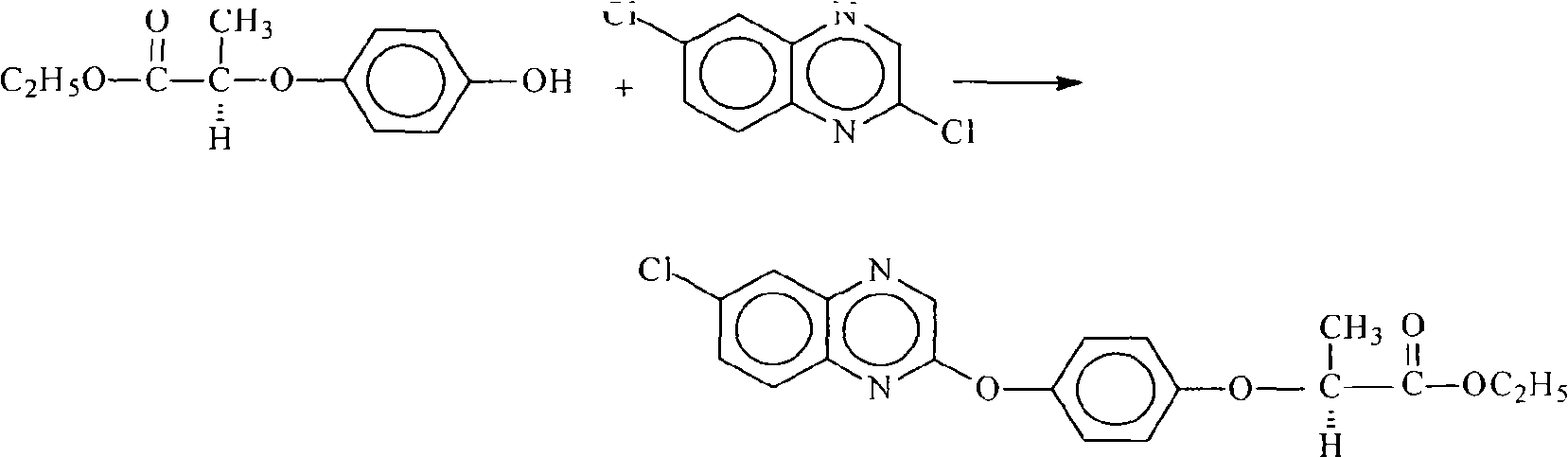
The invention relates to a preparation method for quizalofop-p-ethyl, in particular to a method which uses p-hydroxybenzaldehyde to replace the raw material hydroquinone used in the traditional technology and react with S (-)-N-tosyl ethyl lactate to prepare R (+)-2-(p-oxy) propionate which is then reacted with 2,6- dichloro-quinoxaline to prepare high-purity quizalofop-p-ethyl through condensation.
Owner:SHANDONG CHAMBROAD HLDG GRP CO LTD
Method for preparing ethyl levulinate based on catalysis of alcoholization of furfuryl alcohol with carbon-based solid acid
ActiveCN103288643AEasy to makeLow costPhysical/chemical process catalystsOrganic compound preparationPropanoic acidSolid acid
The invention relates to a method for preparing ethyl levulinate based on catalysis of alcoholization of furfuryl alcohol with carbon-based solid acid, and relates to ethyl levulinate. The method comprises the following steps: uniformly mixing furfuryl alcohol, anhydrous ethanol and the carbon-based solid acid; adding a mixture obtained from the former step into a stainless steel high pressure reaction kettle; closing the reactor; and catalyzing the alcoholization of the furfuryl alcohol so as to prepare ethyl levulinate. The condition for catalysis of the alcoholization of the furfuryl alcohol is as follows: the reaction temperature is 100-150 DEG C, the stirring rotating speed is 300-500rpm, and the reaction time is 0.5-2.5 hours. The weight ratio of furfuryl alcohol to ethanol is (0.01-0.1):1, and the mass ratio of furfuryl alcohol to carbon-based solid acid is (1-4):1. The carbon-based solid acid can be hydrothermal carbon-based solid acid or carbonized carbon-based solid acid. The carbon-based solid acid is used as a catalyst which is simple to prepare and low in cost, and can be repeatedly used, so that the product yield is high and the byproducts are fewer. The method is simple in reaction system, mild in reaction condition, short in reaction time, environment-friendly, low in cost and convenient to posttreat.
Owner:XIAMEN UNIV
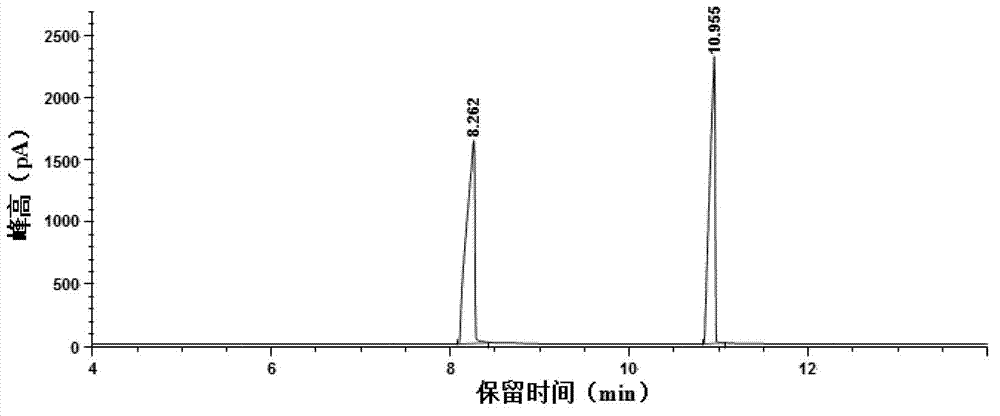
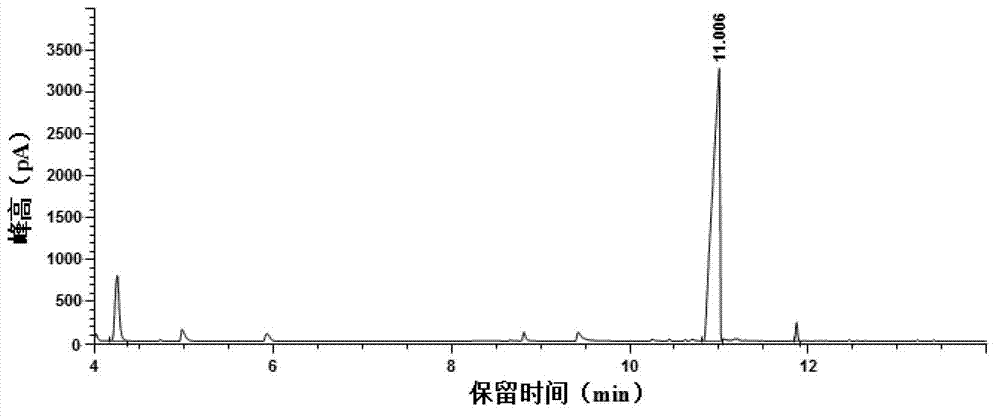
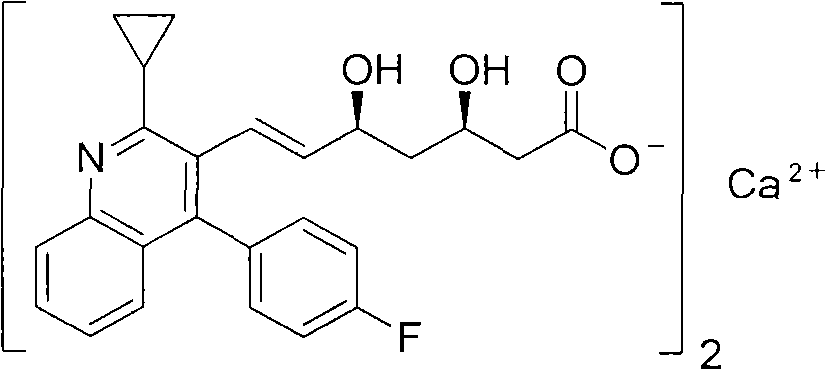
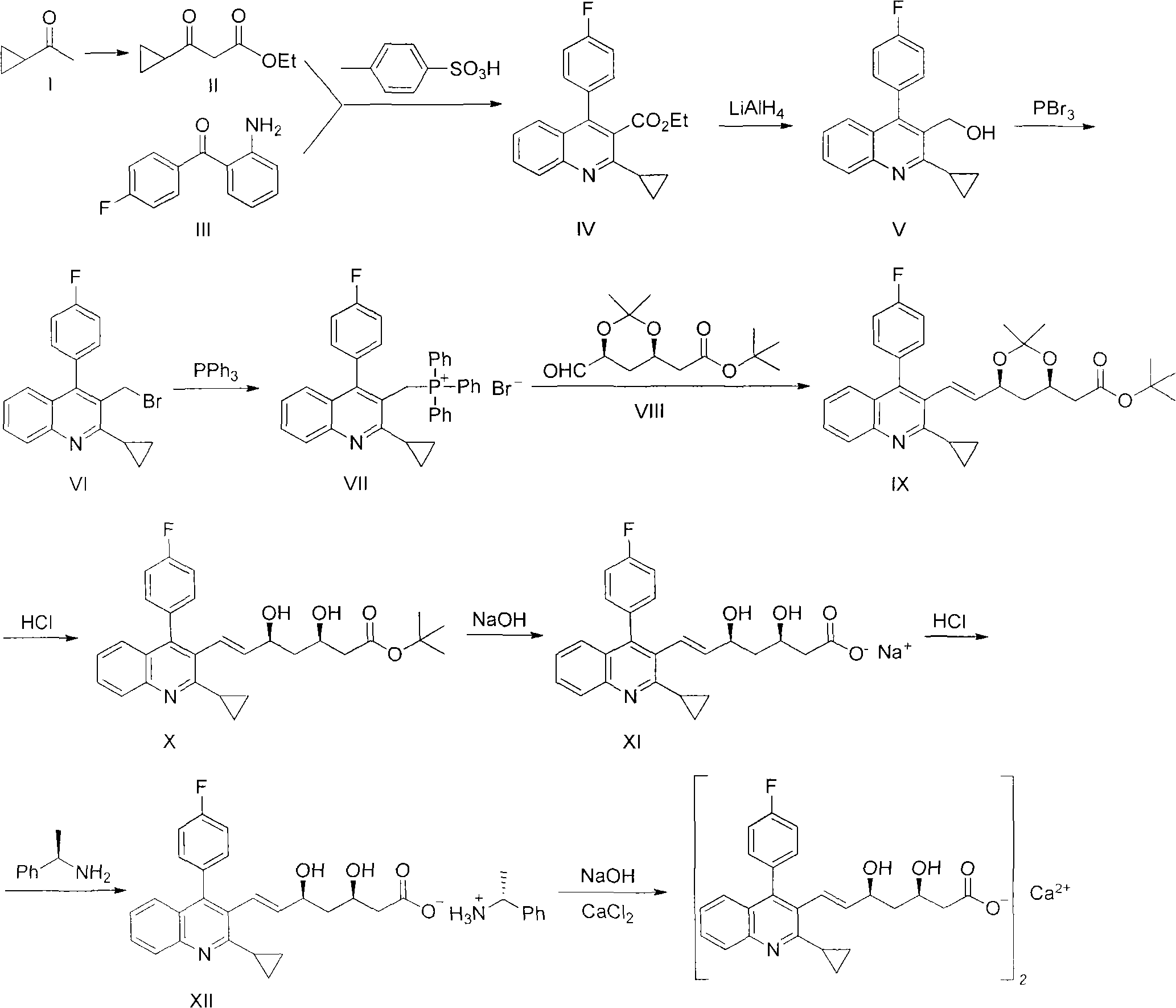
Preparation method of pitavastatin calcium
InactiveCN103508947AReasonable designSimple post-processingOrganic chemistryPhosphonium saltPhosphonium
The invention relates to a preparation method of pitavastatin calcium for treating hyperlipidemia. The preparation method comprises the following steps: performing cyclization on 2-amino-4'-fluorobenzophenone (III) and ethyl 3-cyclopropyl-3-oxo-propanoate (II), and then reducing with LiAlH4 to obtain 2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline methanol (V); bromizing the V to obtain 2-cyclopropyl-3-bromomethyl-4-(4-fluorophenyl) quinoline (VI); reacting the VI with triphenylphosphine to obtain (2-cyclopropyl-4-(4-fluorophenyl)-quinoline-3-yl) methyltriphenylphosphonium bromide (VII), performing alkali treatment on a phosphonium salt, then forming phosphonium ylide, performing condensation with (3R, 5S)-6-oxo-3, 5-isopropylidene-dioxo-6-heptenoic acid tert-butyl ester (VIII) to obtain a compound IX; and acidifying the compound IX with hydrochloric acid, performing hydrolysis deprotection with sodium hydroxide, performing salt formation and purification with chiral amine and forming a calcium slat to obtain a final product. The whole route has reasonable design, the process flow is simple, starting raw materials and reagents used by the preparation method can be purchased from the market, the reagents with severe toxicity and serious pollution are not used in a reaction process, the post-treatment of an intermediate is simple, and the preparation method has the advantages of high yield and is easy to purify.
Owner:WEIHAI WEITAI PHARMA TECH DEV
Method for purifying acrylic acid
The invention relates to the process for purifying acrylic acid which comprises using novel organic extracting agent to separate acrylic acid from its water solution, then removing light compositions such as water and acetic acid by azeotropic fractional distillation, wherein the organic extracting agent comprises toluene, acetic ether, butyl acetate, butyl acetate, propionic ether, diisobutyl ketone, isophorone, methyl phenyl ether or their mixture, preferably the composite compound of toluene with isobutyl phenylacetate, the mass ratio of toluene and isobutyl phenylacetate is between 1:1-1:50, preferably 1:2-1:12. The process according to the invention can realize high acetic acid removing ratio.
Owner:SHANGHAI HUAYI NEW MATERIAL
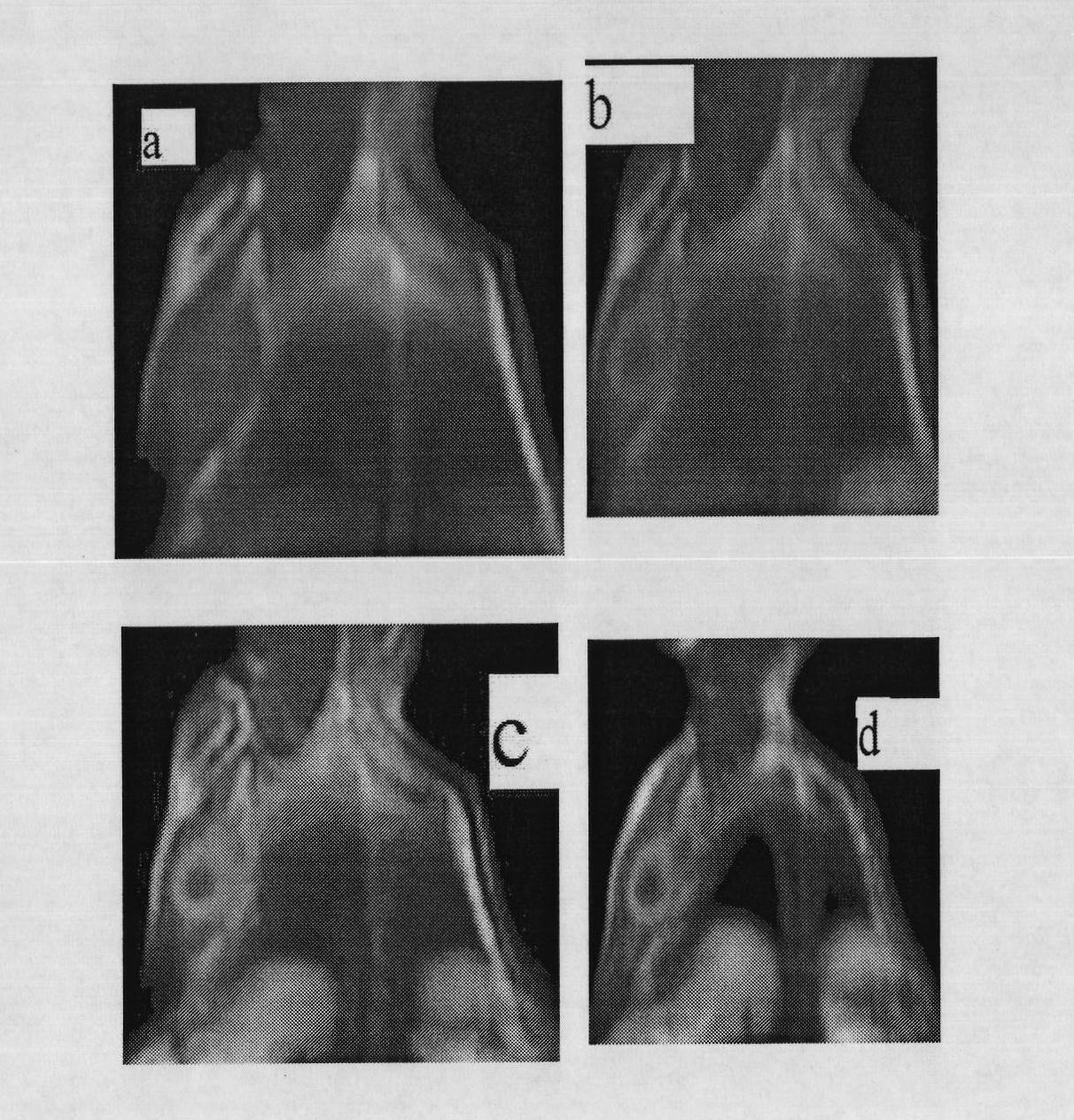
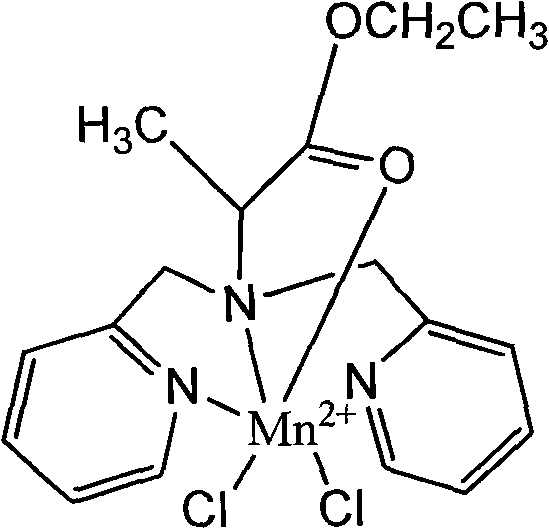
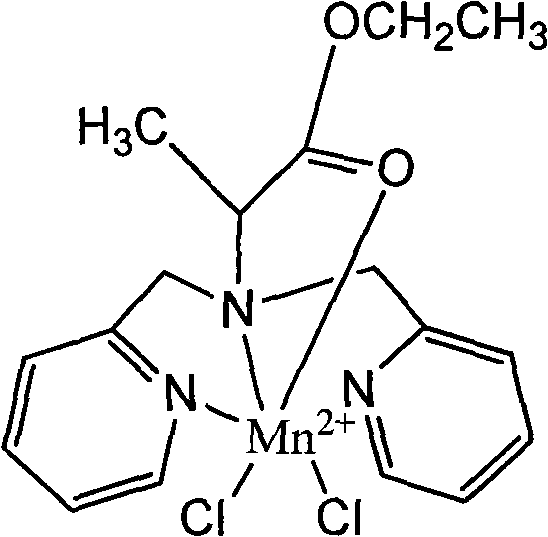
Manganese compound and preparation method and application thereof
InactiveCN101812092AImproving the effect of tumor diagnosisFunction increaseOrganic active ingredientsNMR/MRI constrast preparationsTumor targetingManganese
The invention discloses a manganese compound and a preparation method and application thereof, and relates to the technical field of magnetic resonance imaging (MRI). The manganese compound is dichloro-N-(2-propionic carbethoxy)-N,N-bis(2-picolyl) amine synthetic manganese, and the molecular formula is C17H21Cl2MnN3O2. The preparation method for the manganese compound comprises the following steps: dissolving ligand dichloro-N-(2-propionic carbethoxy)-N,N-bis(2-picolyl) amine and MnCl2 into aqueous solution in a molar ratio of 1 to 1, and controlling the temperature to be between 20 and 40 DEG C; and synthesizing ammonium chloride and a catalyst in a molar ratio of the ammonium chloride to the MnCl2 of 0.2 to 1, reacting the mixture at the temperature of 30 DEG C for 1 to 4 hours, purifying a product by using silica gel column chromatography, concentrating the product and removing the solution to obtain light yellow manganese compound. The manganese compound is applied in the aspect of magnetic resonance imaging in a liver cancer body; and compared with the blank aqueous solution without the manganese compound, the compound can remarkably improve the tumor diagnosis effect, has magnetic resonance imaging function and tumor targeting property, and can be used as a magnetic resonance imaging contrast agent for the tumor.
Owner:JIANGSU UNIV
Carbonyl reductase gene, codase, vector, strain and application of gene
The invention discloses a carbonyl reductase derived from Rhodosporidium toruloides ZJB14212, a gene, a vector, a strain and an application of the carbonyl reductase in the preparation of chiral drug intermediates. The above carbonyl reductase can biologically catalyze preparation of highly optically pure (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propionamide, (S)-N-methyl-3-hydroxy-3-(2-thienyl)propionamide, (R)-3-chloro-1-(2-thienyl)1-propanol, ethyl (S)-3-hydroxy-3-(2-thienyl)propanoate, (S)-3-hydroxy-3-(2-thienyl)propionitrile, tert-butyl 6-chloro-(3R,5S)-dihydroxyhexanoate, (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxyvaleryl]-4-phenyl-1,3-oxazolidine-2-one and methyl [S-(E)]-2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-hydroxypropyl]benzoate.
Owner:ZHEJIANG UNIV OF TECH
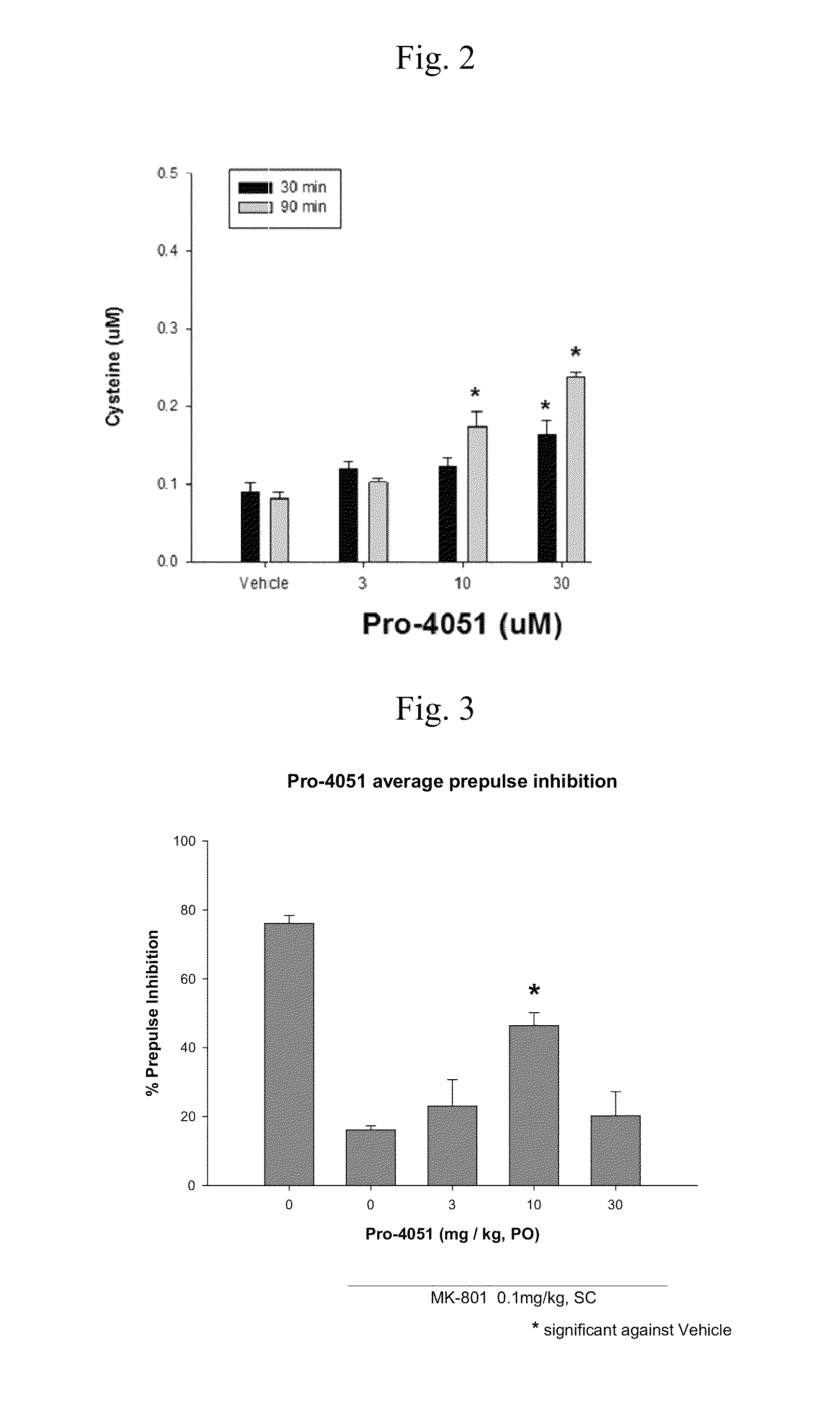
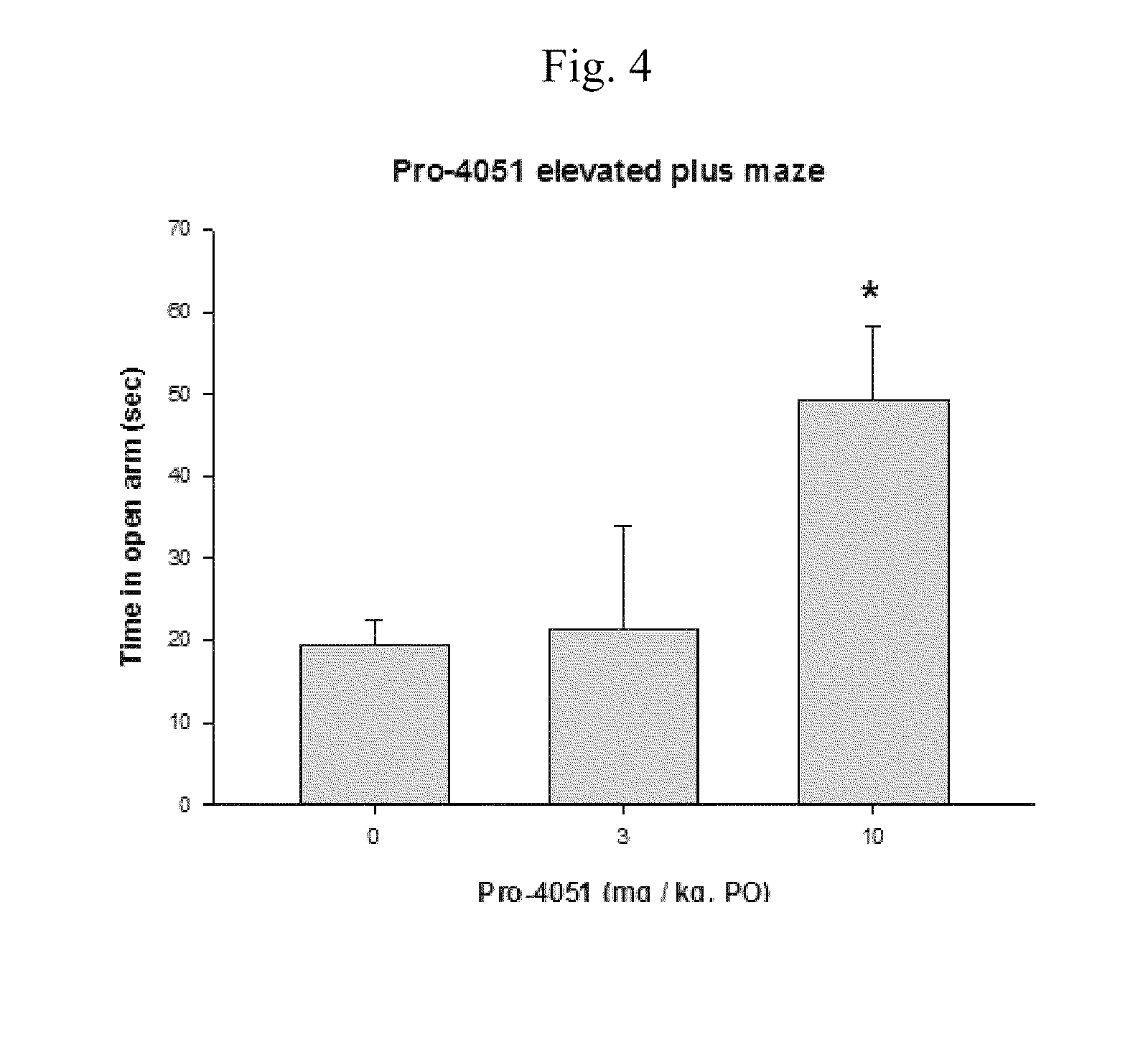
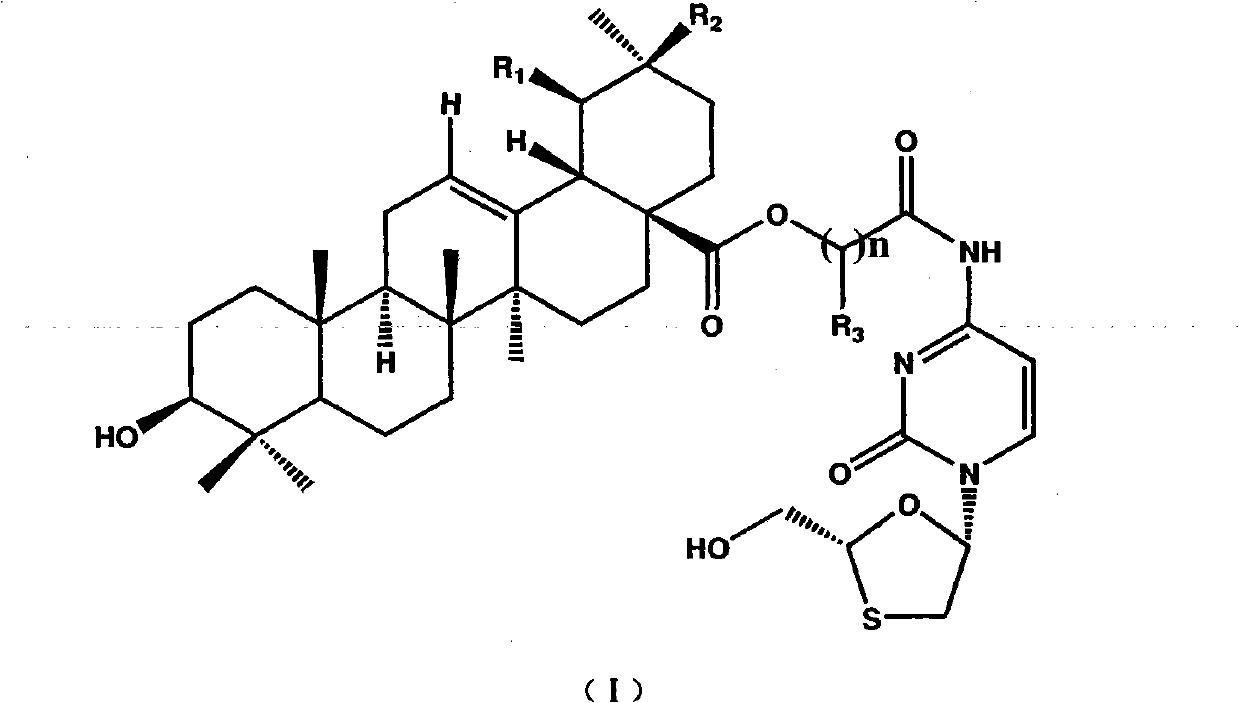
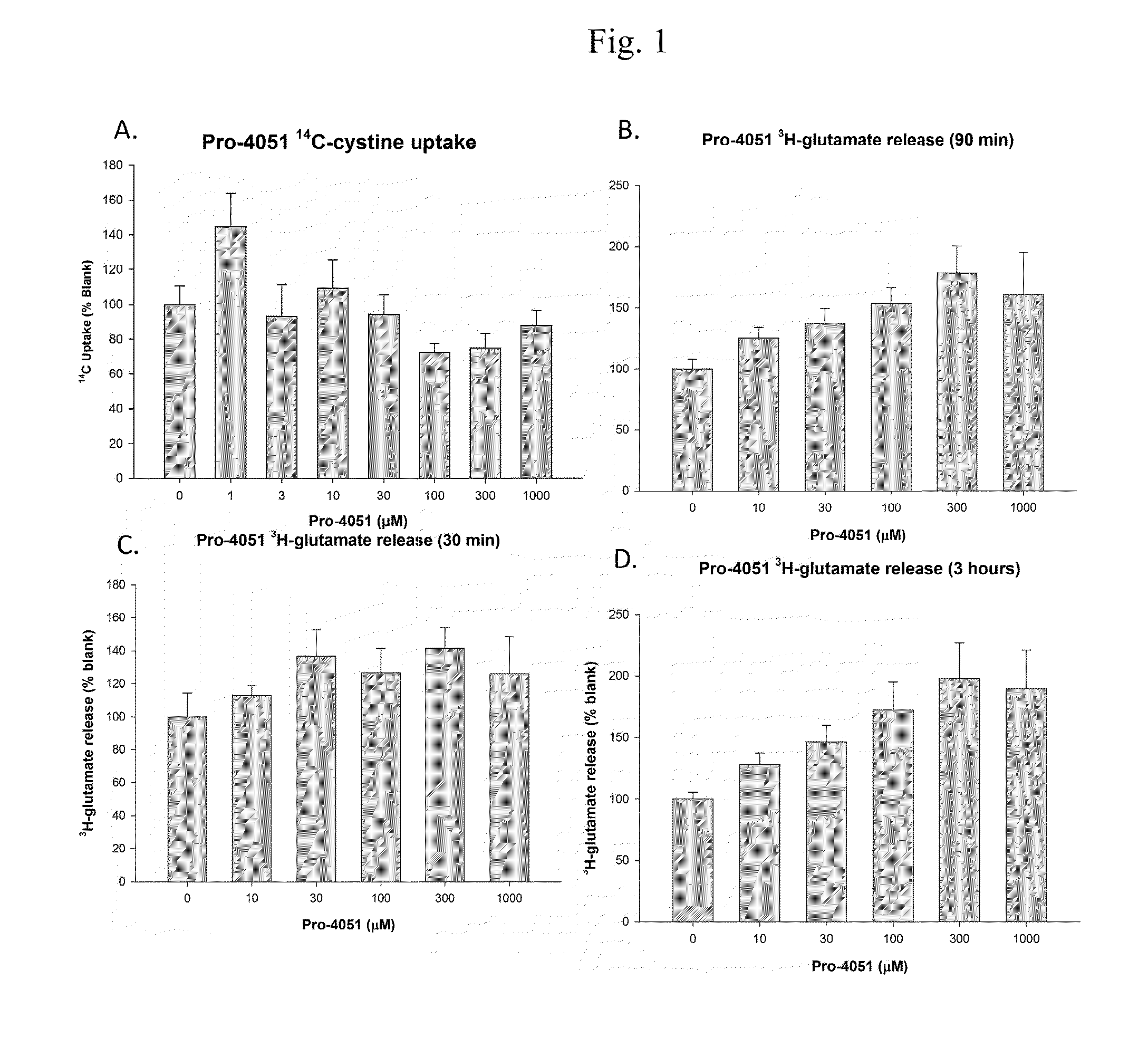
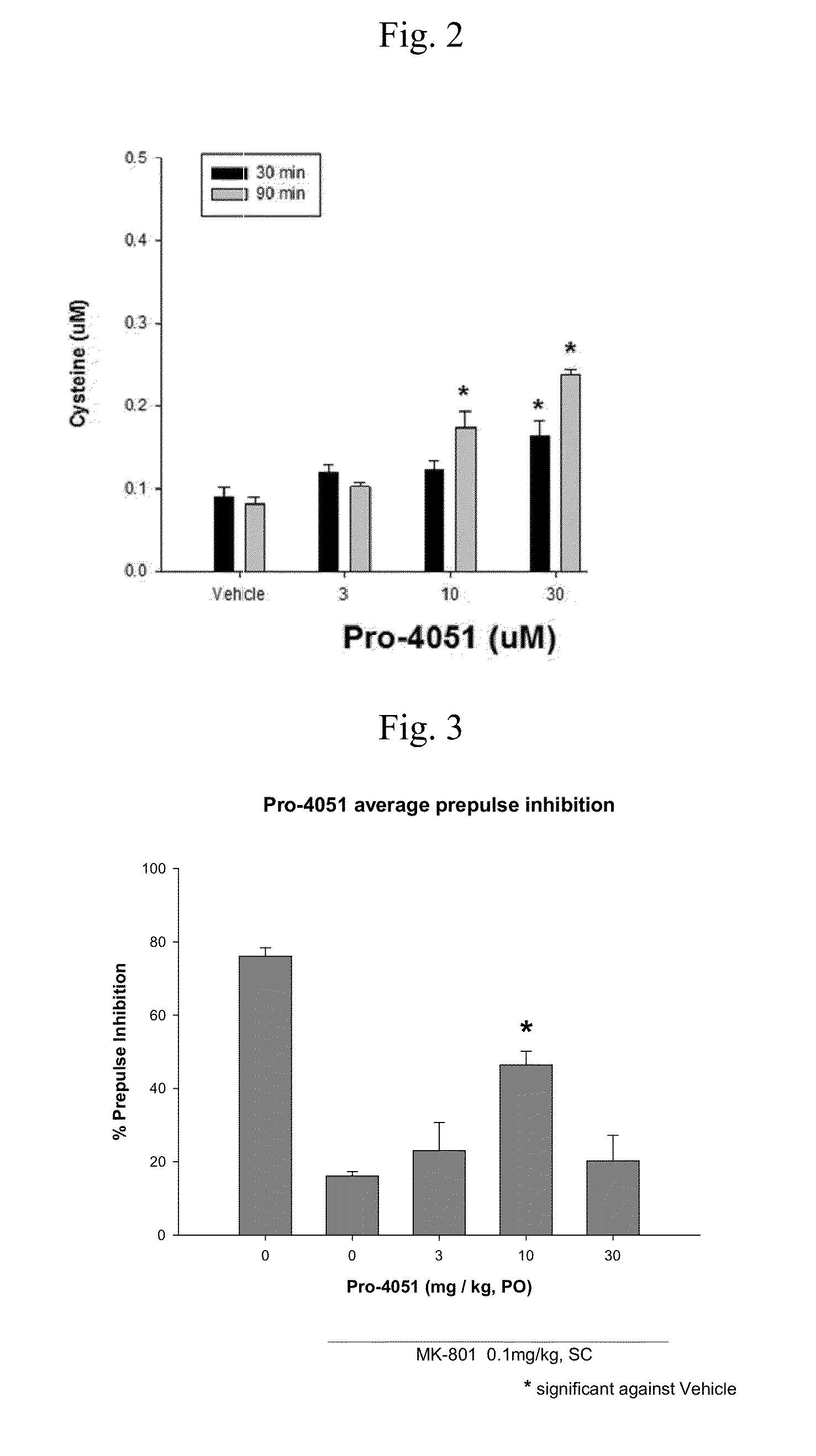
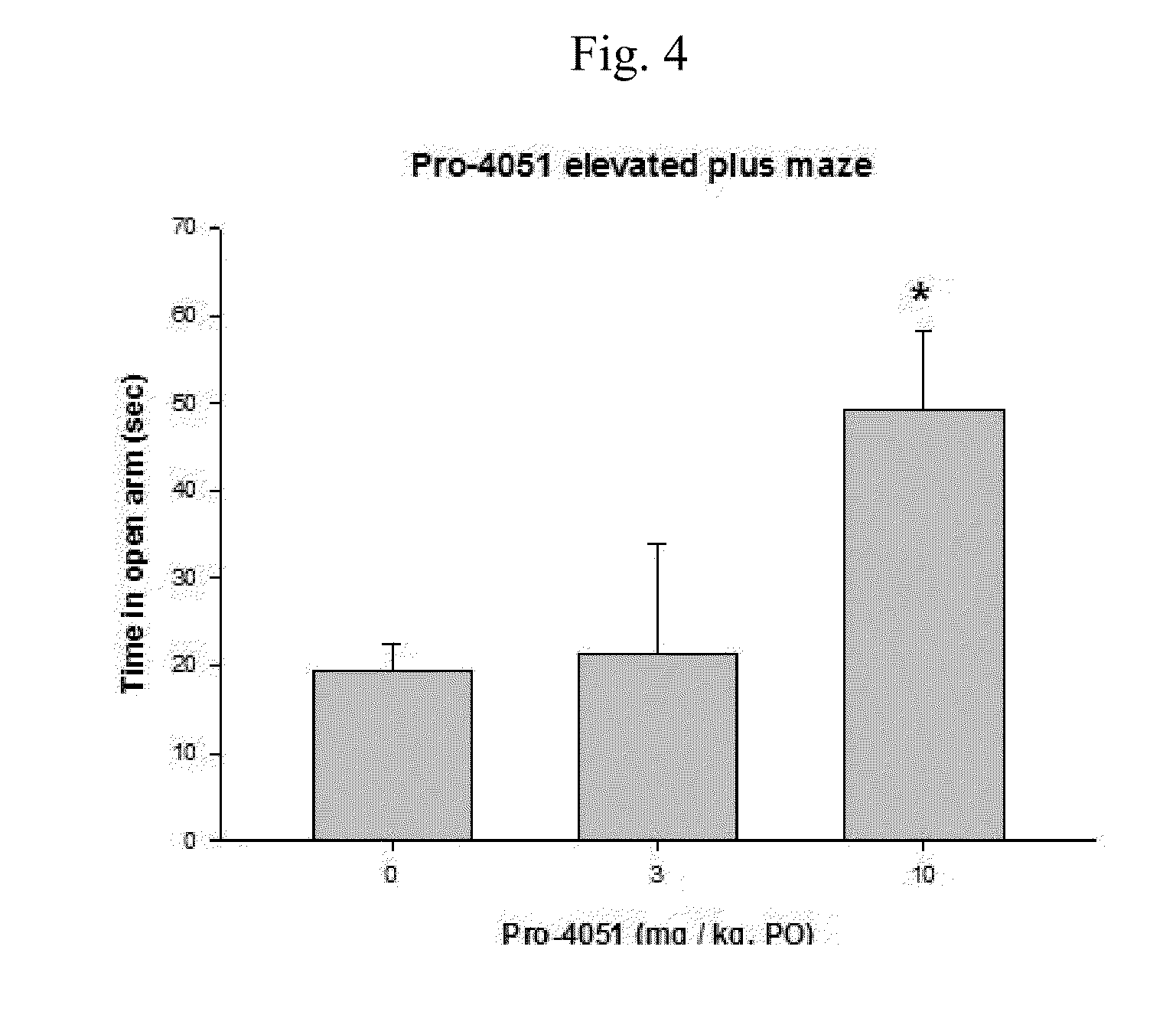
Ethyl (2R)-2-acetamido-3-(4-methylbenzoylsulfanyl)propanoate and uses thereof
A novel substituted N-acetyl-L-cysteine (NAC) derivative and methods of using this compound for the treatment of diseases and / or conditions, including but not limited to diseases and / or conditions of, or involving, the Central Nervous System (CNS), including schizophrenia adrenoleukodystrophy, mitochondrial diseases (e.g. Leigh syndrome, Alpers' disease, and MELAS), Huntington's disease, trichotillomania, HIV-associated neurocognitive disorder, hypoxic-ischemic encephalopathy, drug craving, and drug addiction.
Owner:PROMENTIS PHARMA
Method for preparing valerolactone through catalytic hydrogenation of ethyl levulinate
ActiveCN107245065AEasy to useReduce the hassle of re-preparationOrganic chemistryPhysical/chemical process catalystsTitanium zirconiumMicrosphere
The present invention discloses a method for preparing valerolactone through catalytic hydrogenation of ethyl levulinate. The method comprises: placing ethyl levulinate and different molar ratios of titanium zirconium microsphere catalysts in a device, adding isopropanol as a solvent and a hydrogen source, and carrying out a reaction for 2-10 h at a temperature of 160-200 DEG C, wherein a molar ratio of the titanium zirconium microsphere catalyst is Ti / Zr of 1:0, 8:2, 5:5, 2:8, and 0:1. According to the present invention, the catalytic activity of the method is good, the ethyl levulinate can be completely converted, the yield of the valerolactone can achieve 90.1%, the production cost is reduced by using the alcohol as the hydrogen source, and the catalytic system can be recycled more than 6 times while the catalytic efficiency is not significantly reduced.
Owner:GUIZHOU UNIV
Process for synthesizing methyl acetic acid catalyst by ethanol carbonylation
InactiveCN1736606AWill not damage the skeleton structureHigh specific surface areaCatalyst activation/preparationCarboxylic preparation from carbon monoxide reactionBone structurePropanoic acid
The invention discloses a method for preparation of propionic acid with alcohol by carbonylation. The pretreating technique to active carbon is: treating with deionized water, drying, treating with non- organic acid and cleaning with deionized water to make it neutral, and drying. The catalyst which is supported by metallic salt solution several times is burnt in 400- 600Deg. C high temperature and in a condition of reducing gas in order to be used in the reaction process. Dissolving part soluble material in the active carbon, and none destroying the bone structure of the active carbon, it improves specific surface area and absorption ability of the active carbon, and improves the conversion rate of alcohol and the total yield of propanoic and acid propionic ether.
Owner:SHANGHAI WUJING CHEM
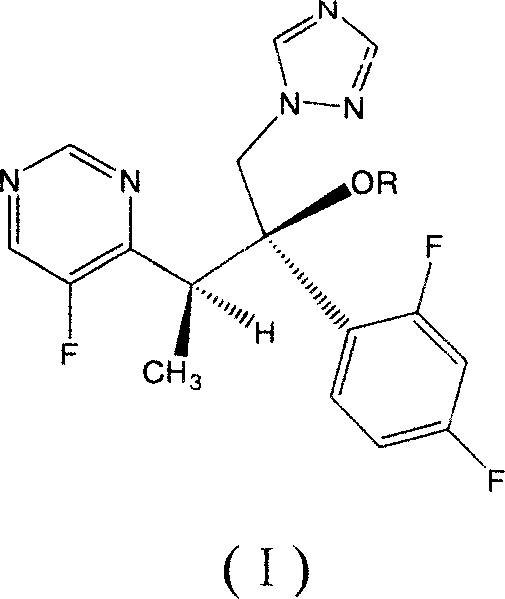
Voriconazole derivate and preparation process thereof
InactiveCN100999518ATo achieve asymmetric synthesisHigh yieldOrganic active ingredientsAntimycoticsPropanoic acidEnantiomer
This invention involves Voriconazole derivatives. The invention also involves Voriconazole derivatives preparation methods, including the following steps : 2 - (5-fluoro-4-yl) acetate and ethanol for esterification, generate 2 -(5-fluoro-4-yl) ethyl acetate, in alkaline conditions takes reaction with methylation agent, generate 2 - (5-fluoro-4-yl) ethyl propionate; hydrolysis of 2 - (5-fluoro-4-yl) propionic acid, through split gain S-2 - (5-fluoro-4-yl) propionic acid; for chlorination to gain S-2 - (5-fluoro-4-yl) propionyl chloride; occurred Friedel-Crafts reaction, generating S-1 - 2, 4-difluoro-2 - (5-fluoro-4-yl) acetone; with 1-methyl - 1-H-1 ,2,4 - triazol under alkali conditions to take reaction to gain voriconazole and the series of voriconazole derivatives. The methods described in this invention has short routes, only use of a pair of enantiomers separation, the overall yield has been greatly improved, is a simple and easy method for synthesis of voriconazole and its derivatives.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Preparation method and applications of lamivudine twin drug
InactiveCN101766632AHas antiviral effectImprove liver functionOrganic active ingredientsDigestive systemHepatic inflammationPropanoic acid
The invention provides a preparation method and applications of a lamivudine twin drug. Lamivudine, and ursolic acid or oleanolic acid are condensed into the lamivudine-ursolic acid or lamivudine-oleanolic acid twin drug at a low temperature by using ethyl chloroacetate (ethyl bromoacetate) or ethyl chloropropionate (ethyl bromopropionate) as the linking group. The twin drug possibly has a dual-action mechanism; and the twin drug has the action of antivirus, and also has the actions of resisting inflammations, protecting the liver cell membranes, improving the liver function and resisting fibrosis. Thus, the invention organically combines the antivirus therapy and the liver protection treatment and provides a new concept for the research and development of drugs for treating hepatitis.
Owner:CHINA PHARM UNIV
Production technology for synthesis of ethyl 2-bromopropionate
InactiveCN103804191AEasy to useEasy to preparePreparation from carboxylic acid halidesOrganic compound preparationBromineChloride
The invention discloses a production technology for synthesis of ethyl 2-bromopropionate and relates to the technical field of chemical engineering. The 2-bromopropionyl chloride is prepared by steps of carrying out the acylating chlorination reaction for sulfoxide chloride, propionic acid, red phosphorus and dry bromine, and then carrying out esterification reaction between the 2-bromopropionyl chloride serving as a raw material and anhydrous ethanol to obtain the ethyl 2-bromopropionate, filtering and distilling. The production technology disclosed by the invention has the beneficial effects that the preparation is convenient and easy, and environment-friendly without pollution, the raw materials are easily available, the equipment investment is small, the purity is high, the operation is convenient, and the prepared ethyl 2-bromopropionate is good in using effect, safe and reliable.
Owner:ANHUI HUARUN PAINTS
Ethyl (2R)-2-acetamido-3-(4-methylbenzoylsulfanyl)propanoate and uses thereof
A novel substituted N-acetyl-L-cysteine (NAC) derivative and methods of using this compound for the treatment of diseases and / or conditions, including but not limited to diseases and / or conditions of, or involving, the Central Nervous System (CNS), including schizophrenia adrenoleukodystrophy, mitochondrial diseases (e.g. Leigh syndrome, Alpers' disease, and MELAS), Huntington's disease, trichotillomania, HIV-associated neurocognitive disorder, hypoxic-ischemic encephalopathy, drug craving, and drug addiction.
Owner:PROMENTIS PHARMA
METHOD FOR PRODUCING n-PROPYL ACETATE
ActiveUS20110065951A1Deterioration can be suppressedReduce frequencyOrganic compound preparationPreparation by transesterificationAllyl acetatePurification methods
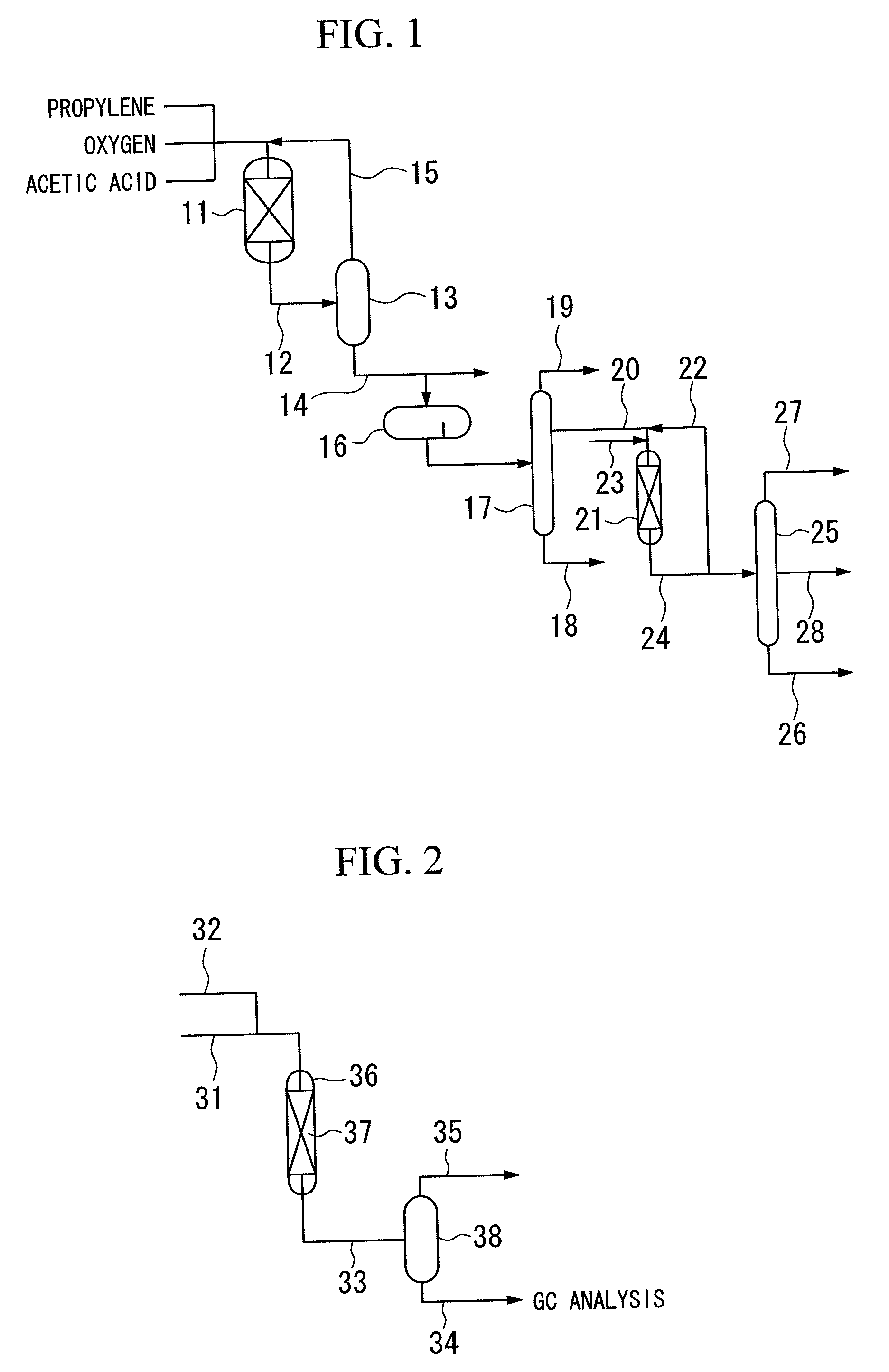
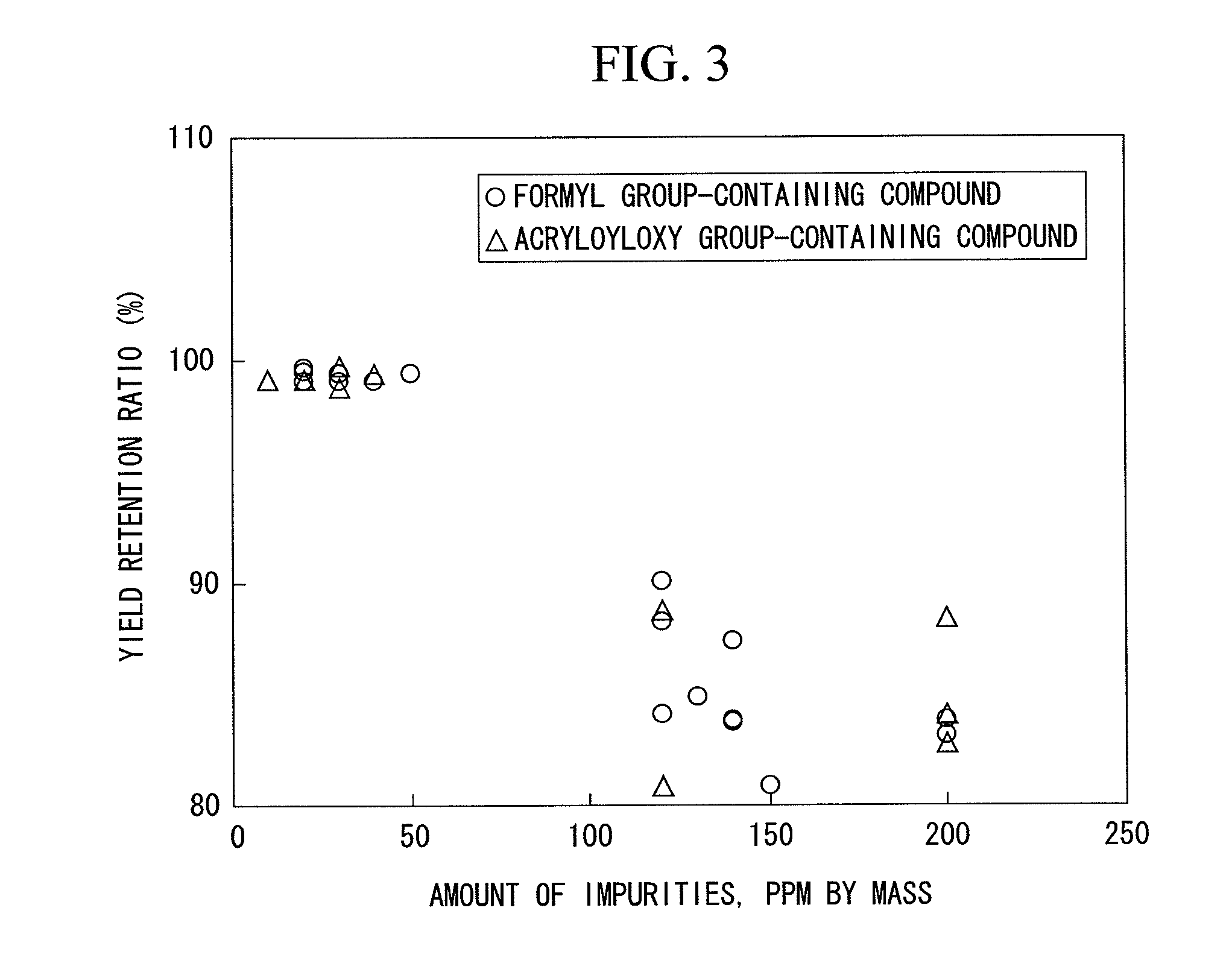
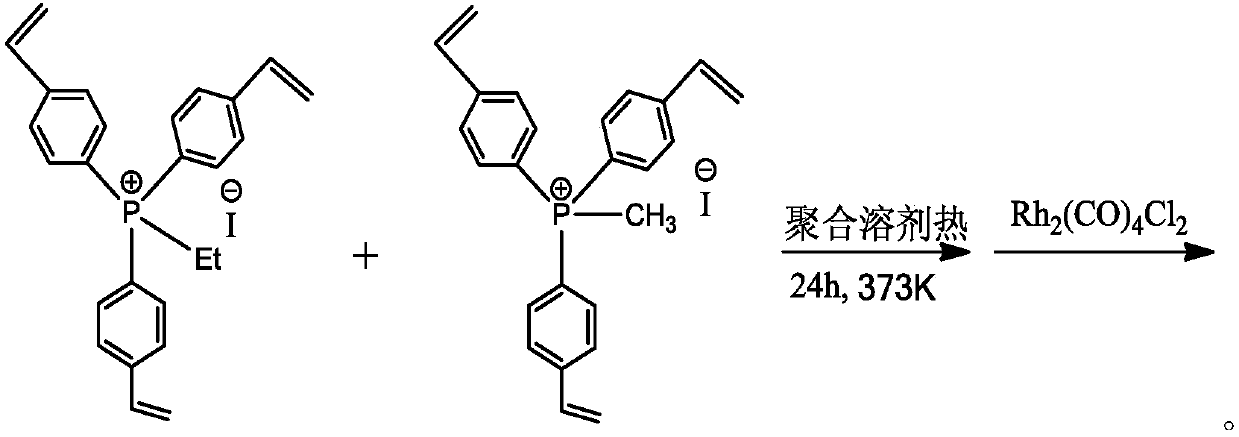
Provided is a method for producing n-propyl acetate capable of suppressing the deterioration of a hydrogenation catalyst to such an extent that the catalyst can be industrially used, for example, in the case where n-propyl acetate is produced by performing a hydrogenation reaction in the presence of the hydrogenation catalyst using, as a raw material liquid, a liquid containing allyl acetate or the like which has been produced from propylene, oxygen and acetic acid and obtained using a purification process such as distillation. In such a method for producing n-propyl acetate, the concentration of a formyl group-containing compound (such as acrolein, propionaldehyde or 2-methylcrotonaldehyde) and the concentration of an acryloyloxy group-containing compound (such as acrylic acid or allyl acrylate) in the raw material liquid are respectively set to 100 ppm by mass or less. As a result, the deterioration of the hydrogenation catalyst can be suppressed to such an extent that the catalyst can be industrially used.
Owner:RESONAC CORP
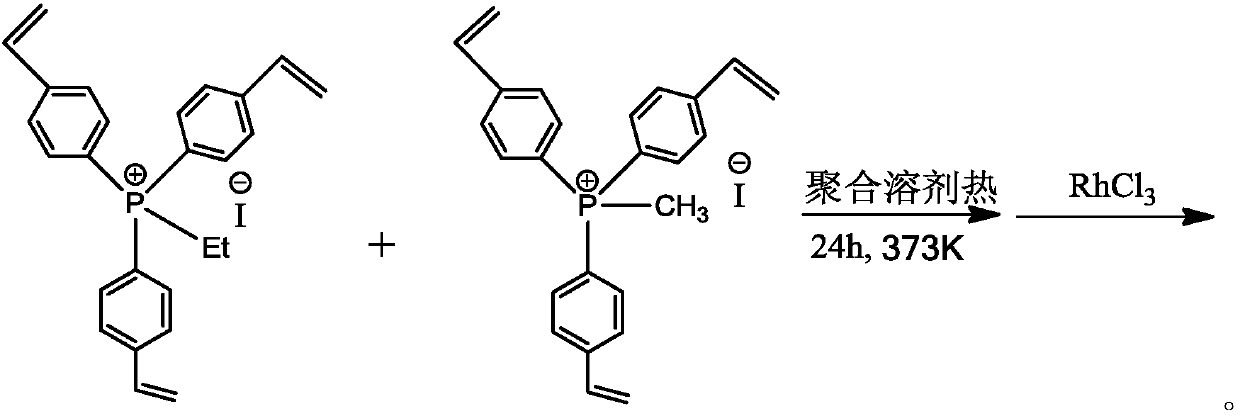
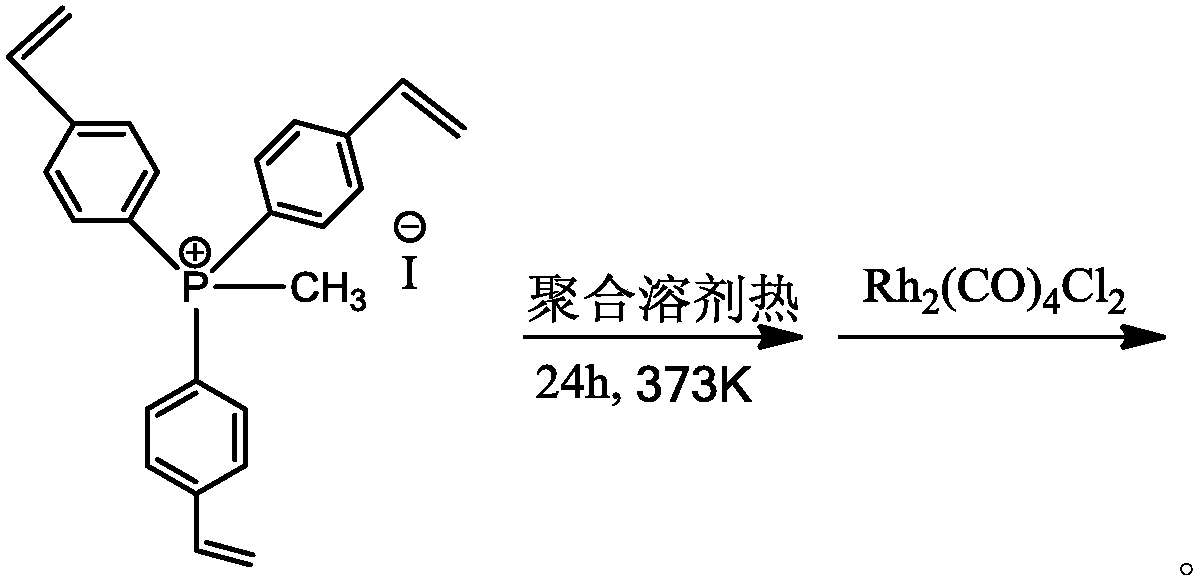
Organic phosphine-containing polymer carrier loaded Rh-based catalyst, preparation and applications thereof
InactiveCN111111775AHigh activityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationPolymer sciencePropanoic acid
The invention provides a large-surface-area hierarchical-pore-structure organic phosphine-containing polymer loaded Rh-based catalyst for preparing ethyl propionate and propionic acid through ethanolheterogeneous carbonylation, and a preparation method thereof. The catalyst is mainly composed of a main active component and a carrier, wherein the main active component is an Rh metal complex, the content of the Rh metal complex is 0.01-5.0% of the weight of the catalyst, the organic phosphine-containing polymer carrier is generated by selecting an organic salt monomer containing vinyl phosphineand adopting a solvothermal method through self-polymerization or mixed polymerization. Through strong ionic bond action between a metal complex and the quaternary phosphonium salt in a polymer carrier skeleton, an Rh metal component is highly dispersed in the polymer carrier in a monatomic form, and in a fixed bed reactor, CH3CH2OH / CO can be converted into ethyl propionate with high activity andhigh selectivity at a certain temperature and pressure under the action of the catalyst and an iodoethane cocatalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
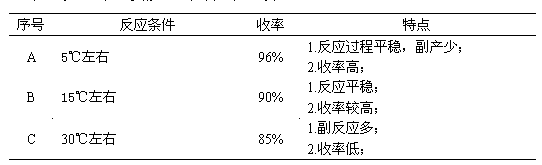
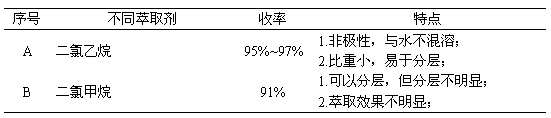
Synthesizing process of 2,3-dicyanoethylpropionate
InactiveCN103214395ANot easy to produceEasy extractionPreparation by cyanide reactionPropanoic acidSodium cyanide
The invention relates to a synthesizing process of 2,3-dicyanoethylpropionate. The process comprises the steps that: electric stirring is started; dimethyl sulfoxide is sucked by using vacuum, and a cooling valve is opened for cooling; ethyl cyanoacetate is sucked; when the temperature is reduced to 4-6 DEG C, paraformaldehyde is added, and sodium cyanide is added; when dosing is finished; heating is carried out for azeotropy; sodium thiosulfate is added, and hydrochloric acid is dropped in, such that a pH value reaches 3-4; a dichloroethane extractant is rapidly added, and the mixture is settled and stratified; an upper-layer material and dichloroethane are delivered to a secondary reaction kettle, and lower-layer water and dimethyl sulfoxide are recovered; the stratified material is pumped into a desolventizing kettle, and dichloroethane is recovered, such that a 2,3-dicyanoethylpropionate crude product is obtained; and rectification is carried out, such that a 2,3-dicyanoethylpropionate finished product is obtained. The process provided by the invention has the advantages that: the synthesizing temperature is controlled at 5+ / -1DEG C; reaction is smooth; side reaction is prevented; and yield is high. The extraction effect of dichloroethane upon the 2,3-dicyanoethylpropionate crude product is better, wherein an extraction rate reaches 97%, such that product yield is greatly improved.
Owner:NANTONG HAISHENG PHARMA
Preparation method of anti-hepatitis C medicine sofosbuvir
ActiveCN111253454ACheap and easy to getHigh puritySugar derivativesSugar derivatives preparationBenzoic acidMethyl benzoate
The invention discloses a preparation method of an anti-hepatitis C medicine sofosbuvir. The method comprises the following steps: taking r-ethyl glycerate acetonide as an initial raw material, enabling the r-ethyl glycerate acetonide to react with ethyl alpha-fluoropropionate under the action of potassium tert-butoxide, performing carbonyl reduction, hydroxyl acylation, hydrolytic cyclization under an acidic condition, hydroxyl acylation, red aluminum reduction and chiral column separation so as to obtain a sofosbuvir key intermediate ((2R,3R,4R,5R)-3-(benzoyloxy)-5-hydroxy-4-fluoro-4-methyltetrahydrofuran-2-yl) methyl benzoate, performing 2-hydroxyl acetylation, enabling acetylized material to react with 2-trimethylsiloxy-4-benzamidopyrimidine, removing benzamido under an acidic condition, performing dehydroxylation protection, and finally enabling obtained material to react with N-[(S)-(2,3,4,5,6-Pentafluorophenoxy)phenoxyphosphinyl]-L-alanine 1-methylethyl ester to obtain the sofosbuvir. The method has the advantages of short synthesis route, high yield, avoidance of a fluorination reaction step in the synthesis process, and mild synthesis reaction conditions.
Owner:IANGSU COLLEGE OF ENG & TECH
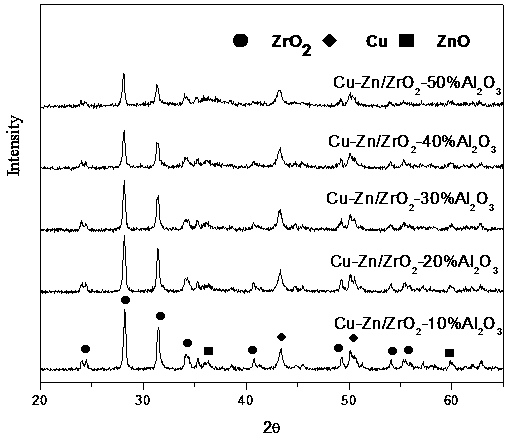
Copper-zinc zirconium oxide metal catalyst and method for catalytic continuous synthesis of gamma-valerolactone by catalyst
InactiveCN107694573AEasy to makeLow costOrganic chemistryCatalyst activation/preparationPtru catalystHydrogenation catalysis
The invention discloses a copper-zinc zirconium oxide metal catalyst and a method for catalytic continuous synthesis of gamma-valerolactone by the copper-zinc zirconium oxide metal catalyst. The method includes the steps: preparing copper-zinc carbonate precipitate according to a co-precipitation method, adding zirconium oxide and aluminum oxide, performing mixed extrusion, drying and calcinationto obtain the copper-zinc zirconium oxide metal catalyst, and catalyzing ethyl levulinate for gamma-valerolactone synthesis by the prepared catalyst subjected to hydrogen reduction. The copper-zinc zirconium oxide metal catalyst taking aluminum oxide as an adhesive is efficient in hydrogenation catalysis of the ethyl levulinate for gamma-valerolactone synthesis and is simple in preparation, low incost, good in catalysis effect and long in service life. Compared with an existing catalyst, the copper-zinc zirconium oxide metal catalyst has the advantages of high selectivity in catalysis of theethyl levulinate for gamma-valerolactone synthesis and fewer side products. In addition, a reaction system is simple, environmentally friendly, low in production cost and capable of continuously synthesizing gamma-valerolactone, and has a good application prospect.
Owner:LIAOCHENG UNIV
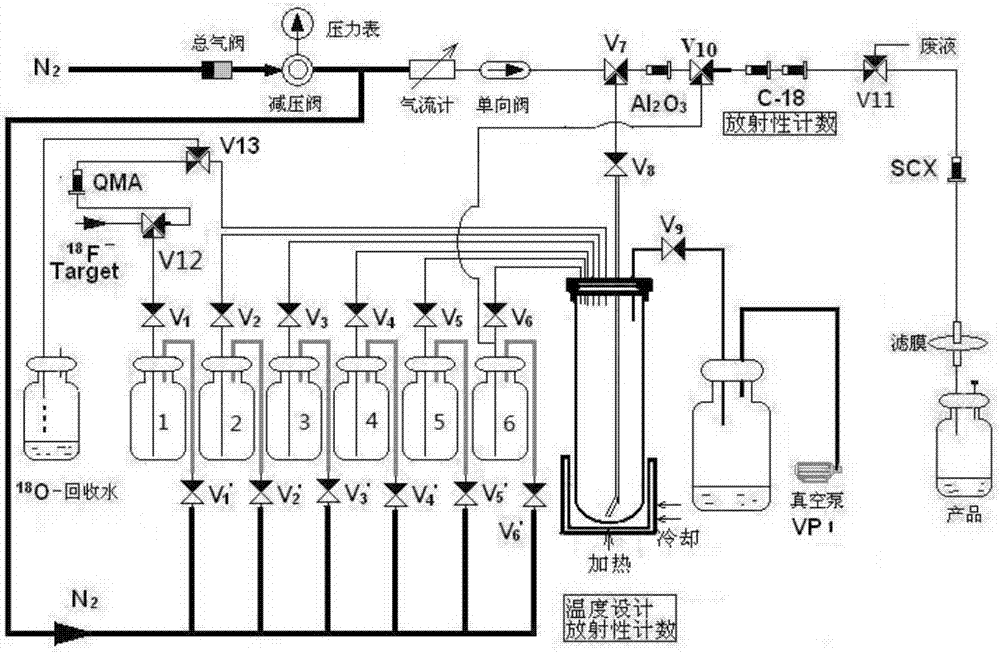
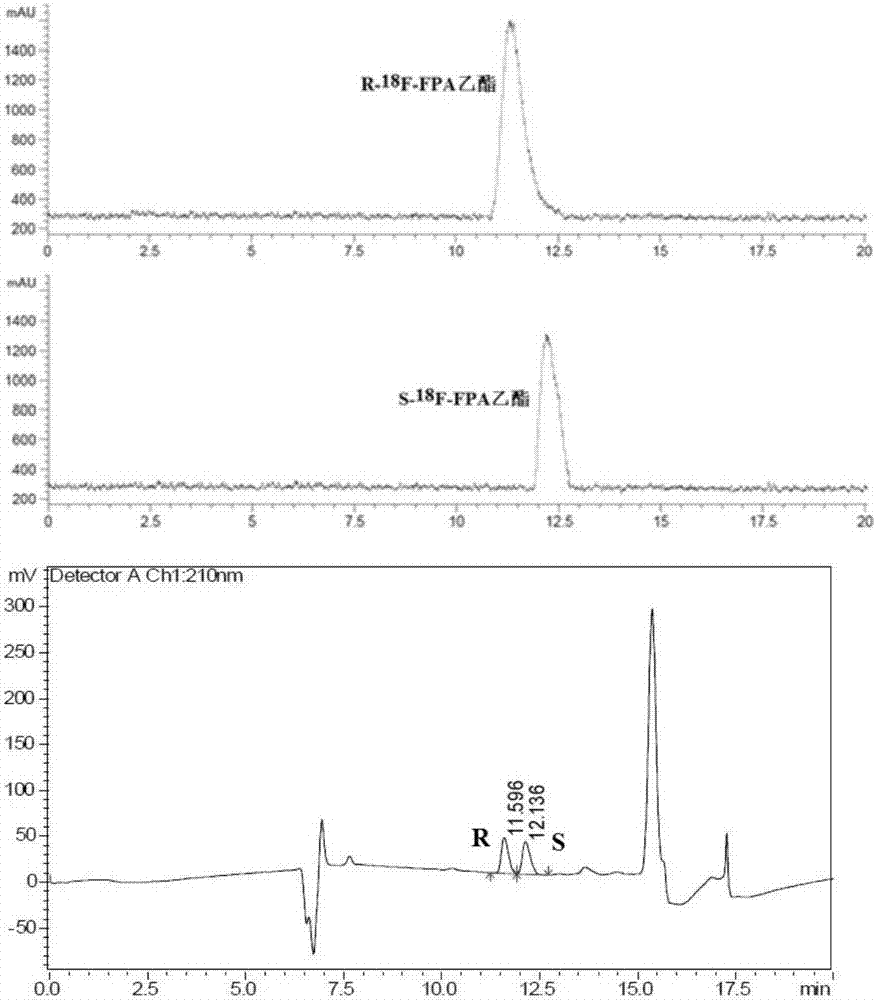
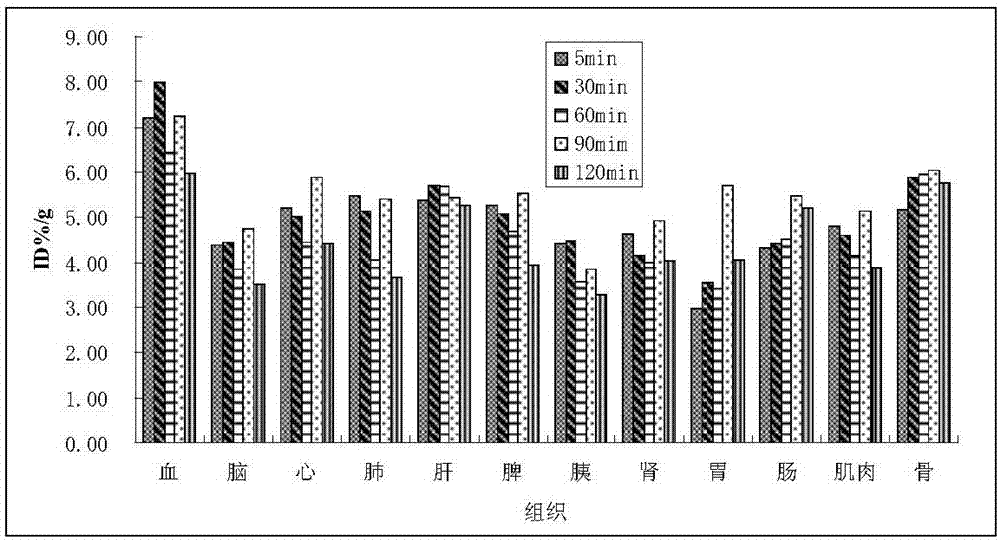
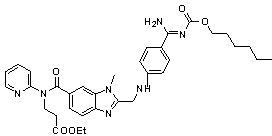
2-<18>F-fluoropropionic acid isomers, synthesis method and application thereof
InactiveCN107118097AOvercome the disadvantage of poor stereoselectivityImproved pharmacokinetic propertiesOrganic compound preparationCarboxylic acid esters preparationDiseaseSynthesis methods
The invention relates to 2-<18>F-fluoropropionic acid isomers: R-2-<18>F-fluoropropionic acid (R-<18>F-FPA, formula 1) and S-2-<18>F-fluoropropionic acid (S-18F-FPA, formula 2), a synthesis method and application thereof in preparation of positron emission tomography (PET) imaging agent drugs. Enantiomer R- or S-2-trifluoromethanesulfonic acid-ethyl propionate is adopted as the precursor, and can be obtained by nucleophilic fluorination and hydrolysis two-step reaction. The chiral PET drug radiation synthesis method provided by the invention is simple, has high radiochemical yield, and is convenient for automatic synthesis, and the enantiomers can be used for differential diagnosis and therapeutic effect evaluation of tumors, cardiovascular and cerebrovascular diseases and neuropsychiatric disorders. (formula 1, and formula 2).
Owner:GUANGDONG HUIXUAN PHARMA TECH
Preparation method of 3-(2-pyridineamino)ethyl propionate
InactiveCN103058920ALow priceEasy to makeOrganic chemistryChemical recyclingPtru catalystPropanoic acid
The invention discloses a preparation method of a 3-(2-pyridineamino)ethyl propionate. The method comprises the following steps: performing catalytic reaction by using 2-aminopyridine and ethyl acrylate as a raw material, and using a Bronsted acid loaded by silicagel as a catalyst in an inorganic solvent or an organic solvent and controlling temperature at 80-120 DEG C through oil bath heating, wherein the temperature of the obtained reaction mothersolution is 40-45 DEG.C, concentrating at the pressure of 0.09-0.1MPa at reduced pressure, performing the chromatography on the concentrated solution through a silicagel column or an aluminum oxide column to obtain 3-(2-pyridineamino)ethyl propionate. The preparation method of the 3-(2-pyridineamino)ethyl propionate has the characteristics of simple preparation method, convenience in operation, low production cost, and short reaction time; the used silicagel-loaded Bronsted acid catalyst can be recycled, and the product yield of the finally obtained 3-(2-pyridineamino)ethyl propionate achieves 50-76%.
Owner:SHANGHAI INST OF TECH
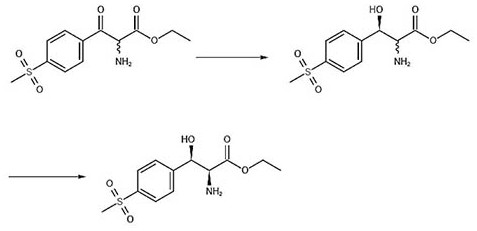
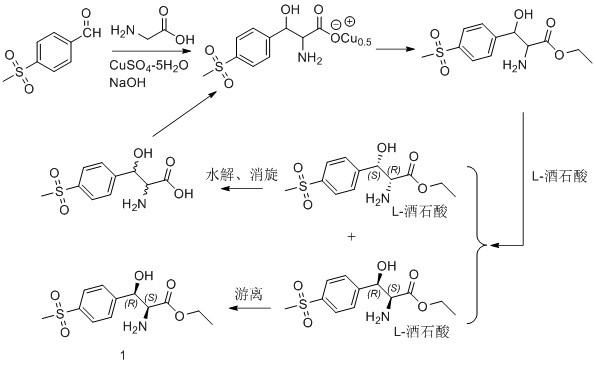
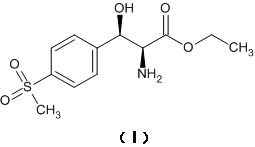
Asymmetric synthesis method for preparing (2S, 3R)-p-methylsulfonyl phenyl serine ethyl ester
ActiveCN114014787AShort process routeHigh optical purityOrganic compound preparationAsymmetric synthesesPropanoic acidKinetic resolution
The invention relates to an asymmetric synthesis method for preparing (2S, 3R)-p-methylsulfonyl phenyl serine ethyl ester. (-)-diisocamphene boron chloride (namely (Ipc) 2BCl) as a chiral reagent and 2-amino-3-[4-(methylsulfonyl) phenyl]-3-oxo-ethyl propionate as a prochiral ketone carbonyl compound react to realize asymmetric reduction of keto-carbonyl groups of the 2-amino-3-[4-(methylsulfonyl) phenyl]-3-oxo-ethyl propionate, preparing (3R)-p-methylsulfonyl phenyl serine ethyl ester, and carrying out kinetic resolution to obtain the (2S, 3R)-p-methylsulfonyl phenyl serine ethyl ester. The process route is short, the optical purity of the product is high (e.e. Value is greater than 98%), and the product yield is high.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Production method of aryl pyrazole nitrile
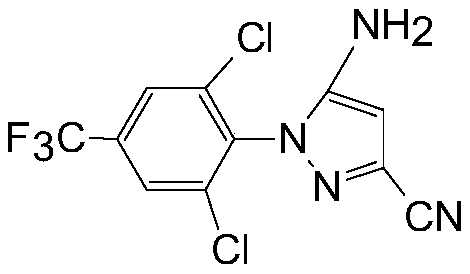
PendingCN111018786AEliminate potential safety hazardsRealize automated productionOrganic chemistryMethylanilineAryl
The invention discloses a production method of aryl pyrazole nitrile, which comprises the following steps: dissolving 2, 6-dichloro-4-trifluoromethylaniline and ethyl 2, 3-dicyanopropionate in an acidic solvent to form a solution, adding the solution and sodium nitrite or a suspension composed of sodium nitrite and an alcohol into a reactor at the same time, and reacting to obtain a synthetic solution 1; adding a reducing agent into the synthetic liquid 1 to terminate the reaction to obtain a synthetic liquid 2; adding an alkaline material into the synthetic liquid 2 to carry out alcoholysis and cyclization under an alkaline condition to obtain a synthetic liquid 3; and adding the synthetic liquid 3 into an acidic solvent for neutralization, desolventizing and refining to obtain a target product.
Owner:江苏优普生物化学科技股份有限公司
Method for preparing propionic acid from ethyl acetate through carbonylation
InactiveCN102911035AOvercome the priceOvercome the disadvantages of increasingCarboxylic preparation from carbon monoxide reactionPropanoic acidIodide
The invention discloses a method for preparing propionic acid from ethyl acetate through carbonylation. A reaction system consists of ethyl acetate, a main catalyst, a co-catalyst and water according to a molar ratio of 1:0.01-0.0001:0.05-0.5:1-10, wherein the reaction temperature is 140-300 DEG C, and the carbon monoxide pressure is 2.0-10.0 MPa; the main catalyst is a rhodium compound; the co-catalyst is iodoethane; an iodide additive such as hydriodic acid, lithium iodide, potassium iodide, tin iodide, lead iodide, zinc iodide or cadmium iodide, a polar solvent such as acetic acid, propionic acid or butyric acid and an organic ligand are selectively added into the reaction system; the molar ratio of the iodide additive to the ethyl acetate is 0.01-1; the molar ratio of the polar solvent to the ethyl acetate is 0.1-10; and the molar ratio of the organic ligand to the main catalyst is 1-5. The process raw materials are freely and economically selected, the problem that ethanol is hydrolyzed and esterified to produce diethyl ether and propionic acid under an acidic condition is solved, and the selectivity of the propionic acid is improved.
Owner:JIANGSU SOPO GRP +1
Method for immobilizing pseudomonas fluorescens lipase by metal organic framework
InactiveCN111471663ANot easy to fall offHigh catalytic activityHydrolasesOn/in organic carrierBiotechnologyO-Phosphoric Acid
The invention discloses an immobilization method of pseudomonas fluorescens lipase, and particularly relates to an immobilization method of lipase for chiral drug separation. The method comprises thefollowing steps of activating Uio-66 (Zr) MOF by using a phosphoric acid buffer solution of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, then adding N-hydroxysuccinimide, and then adding a phosphoric acid buffer solution of the pseudomonas fluorescens lipase into a mixed solution to carry out covalent cross-linking, thus obtaining a final product. According to the enzyme immobilization method, the lipase is immobilized by using a metal organic framework material, so that the activity and enantioselectivity of free enzyme can be maintained, and meanwhile, the lipase has stability and reusability. The immobilized enzyme is used for catalytic separation of a 2-(4-hydroxyphenyl) ethyl propionate enantiomer and a 4-methoxymandelic acid enantiomer, (R)-(-)-2-(4-hydroxyphenyl) propionic acid and (R)-4-methoxymandelic acid are prepared, and high catalytic activity and enantioselectivity are shown; and the method provides the wide prospect for the immobilized enzyme in industrial application.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
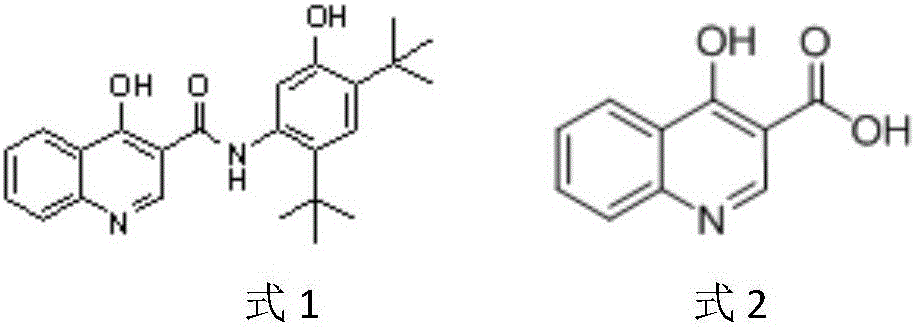
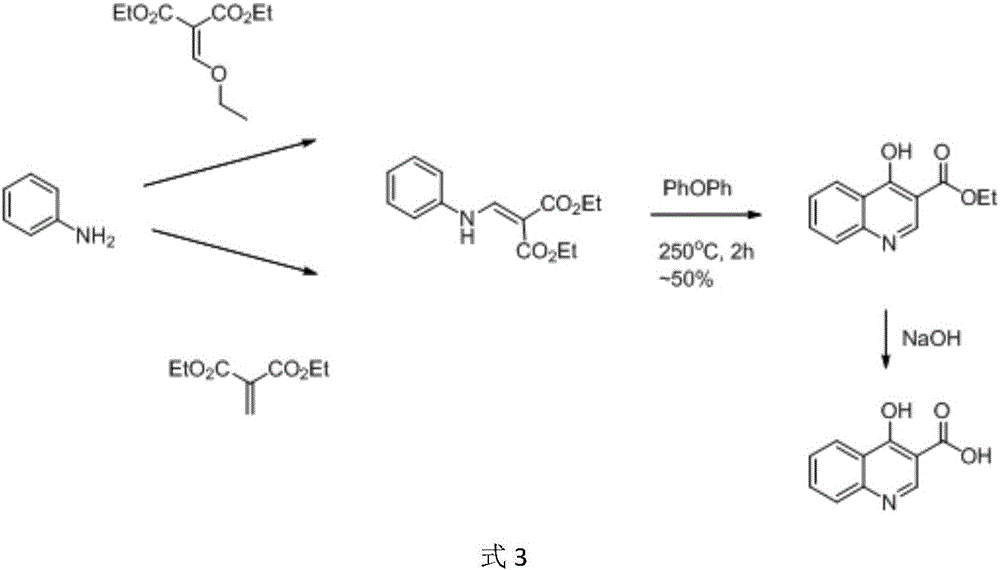
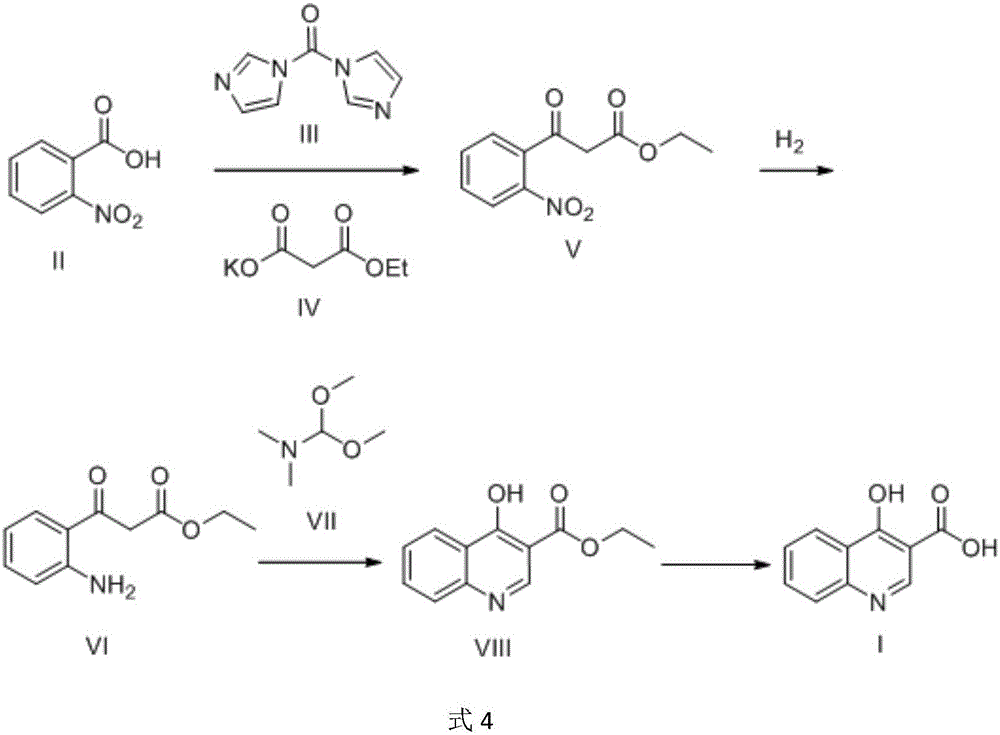
Preparation method of 4-hydroxyquinoline-3-carboxylic acid
ActiveCN106187887ARaw materials are easy to getSimple processOrganic chemistryOrganic synthesisCarboxylic salt
The invention relates to the technical field of organic synthesis and bulk drug intermediates, and concretely relates to a preparation method of a key intermediate 4-hydroxyquinoline-3-carboxylic acid of a new medicine ivacaftor for treating cystic fibrosis. The preparation method comprises the following steps: 1, carrying out a condensation reaction: reacting o-nitrobenzoic acid, potassium monoethyl malonate and N,N-carbonyldiimidazole to prepare ethyl 3-(2-nitrophenyl)-3-oxopropanoate; 2, carrying out a reduction reaction: carrying out catalytic hydrogenation reduction on ethyl 3-(2-nitrophenyl)-3-oxopropanoate to prepare ethyl 3-(2-aminophenyl)-3-oxopropanoate; 3, carrying out a cyclization reaction: carrying out nucleophilic addition and cyclization reaction on ethyl 3-(2-aminophenyl)-3-oxopropanoate and N,N-dimethyl formamide dimethyl acctel to obtain ethyl 4-hydroxyquinoline-3-carboxylate; and 4, carrying out a hydrolysis reaction: carrying out the hydrolysis reaction on ethyl 4-hydroxyquinoline-3-carboxylate to obtain 4-hydroxyquinoline-3-carboxylic acid. The preparation method has the advantages of easily available raw materials, mild reaction conditions, simplicity and convenience in post-treatment, suitableness for amplified preparation, and high yield.
Owner:SHANGHAI UNIV OF ENG SCI
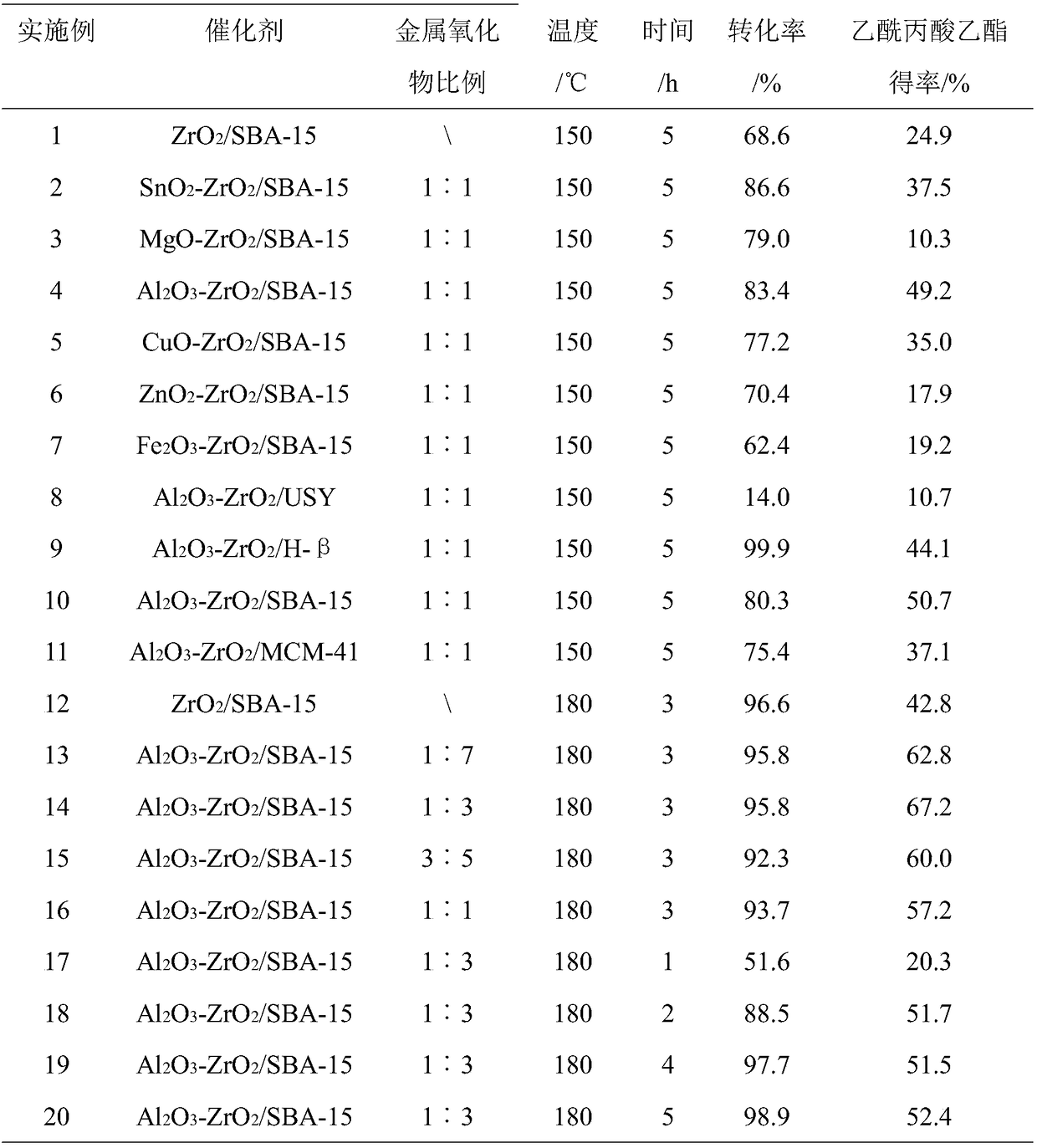
Method for preparing ethyl acetylpropionate by catalyzing furfural via one-pot method
ActiveCN108689837AEasy to recycleEasy to separateMolecular sieve catalystsOrganic compound preparationFurfuralSolvent
The invention relates to a method for preparing ethyl acetylpropionate by catalyzing furfural via a one-pot method, and relates to ethyl acetylpropionate. The method comprises the following steps of heating furfural, a catalyst and ethyl alcohol in a reaction kettle, cooling to room temperature, centrifuging and decomposing, obtaining a liquid phase, and purifying, so as to obtain ethyl acetylpropionate; using the solid as the recycling catalyst. The method has the advantages that furfural is directly used as the raw material to prepare ethyl acetylpropionate; used ethyl alcohol can be simultaneously used as reaction raw material, solvent and hydrogen donor, the external hydrogen source and other solvents are not needed, and the reaction system is simple, so as to conveniently separate thetarget product and recycle and reutilize the solvent; the preparation procedure of a dual-function molecular sieve-loaded catalyst for simultaneously catalyzing the transfer hydrogenation and alcoholysis reaction is simple, the price of the raw material is low, and ethyl acetylpropionate with higher yield rate can be obtained by catalyzing furfural; the used catalyst can be conveniently recycled,and the activity is still stable after recycling.
Owner:XIAMEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com