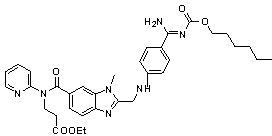
Preparation method of 3-(2-pyridineamino)ethyl propionate
A kind of technology of ethyl propionate and pyridyl amino, applied in the field of preparation of ethyl 3-propionate, can solve the problems of long synthesis steps, long reaction time and high production cost, and achieves low production cost, short reaction time and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of preparation method of 3-(2-pyridylamino) ethyl propionate, the steps are as follows:
[0029] Add 2-aminopyridine (0.47g, 5mmol) and ethyl acrylate (1.0g, 10mmol) to four 25mL round bottom flasks or 15mL sealed tubes, respectively, and then add perchloric acid (0.5 g, 0.5mmol), 200-300 mesh silica gel loaded perchloric acid (0.5g, 0.5mmol), 300-400 mesh silica gel loaded perchloric acid (0.5g, 0.5mmol), silica gel H loaded perchloric acid ( 0.5g, 0.5mmol) as the catalyst, under the condition of ethanol (2mL) as the organic solvent, the reaction temperature was controlled at 100°C for 24h, the reaction mother liquor obtained after the reaction was separated by a centrifuge, the supernatant was taken, and the supernatant The supernatant liquid is concentrated at a temperature of 40-45°C and a pressure of 0.09-0.1 MPa under reduced pressure. The concentrated liquid is passed through a silica gel column or an aluminum oxide column with a ratio of petroleum ether:e...
Embodiment 2
[0032] A kind of preparation method of 3-(2-pyridylamino) ethyl propionate, the steps are as follows:
[0033] Add 2-aminopyridine (0.47g, 5mmol) and ethyl acrylate (1.0g, 10mmol) into a 25mL round bottom flask or a 15mL sealed tube, add perchloric acid (0.5g 0.5mmol) loaded on 200-300 mesh silica gel Catalyst, ethanol (2mL) as solvent, control the temperature at 100°C for 24 hours, separate the reaction mother liquor obtained after the reaction with a centrifuge, take the supernatant, control the temperature of the supernatant at 40-45°C, and the pressure at 0.09- Concentrate under reduced pressure at 0.1MPa, and the concentrated solution is subjected to column chromatography with a silica gel column or an aluminum oxide column and an eluent with a ratio of petroleum ether:ethyl acetate of 8:1 to 5:1, and the collected 5:1 When the eluent, the white solid product obtained by spinning is 3-(2-pyridylamino) ethyl propionate, and the productive rate is 65%;
[0034] The white s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com