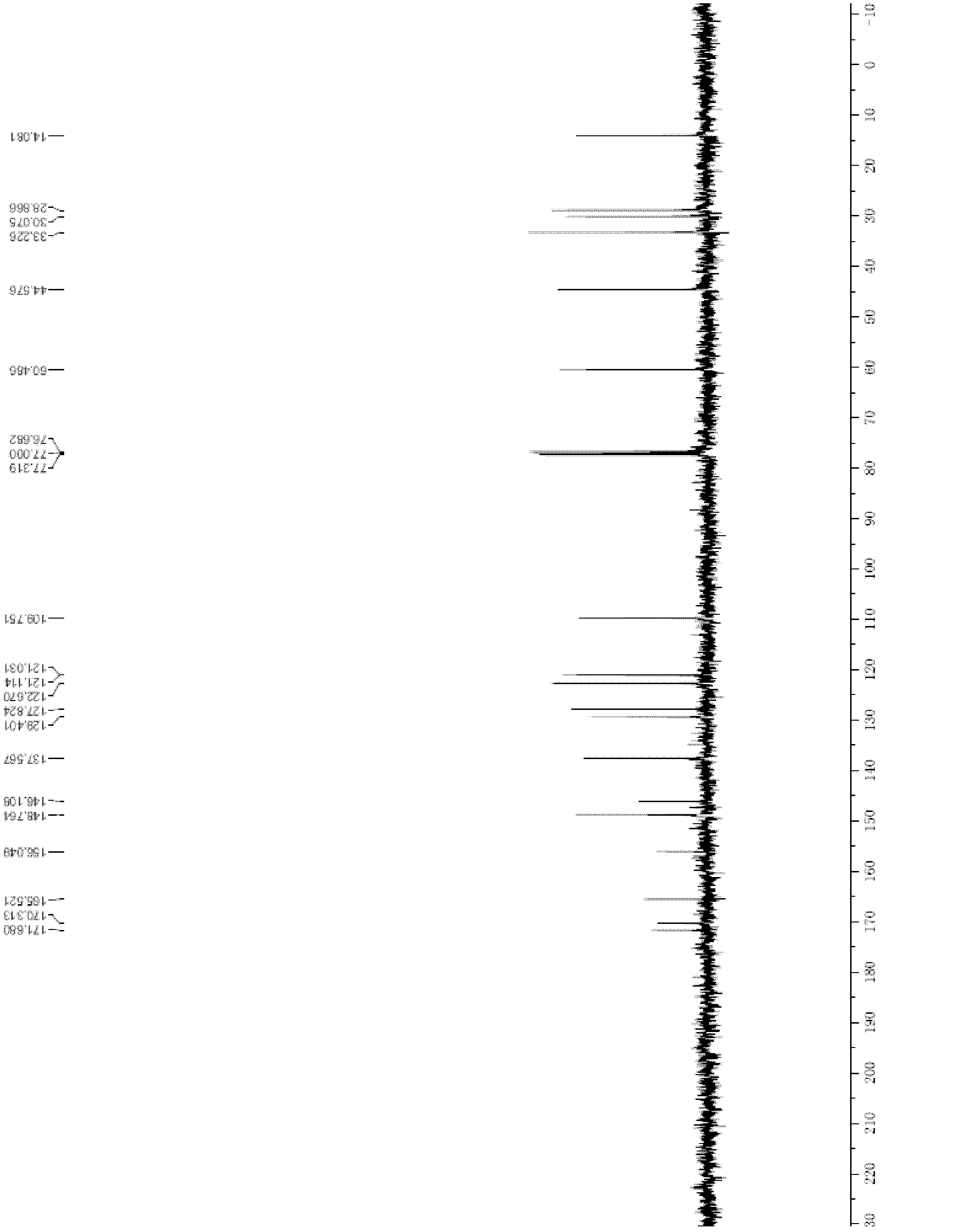
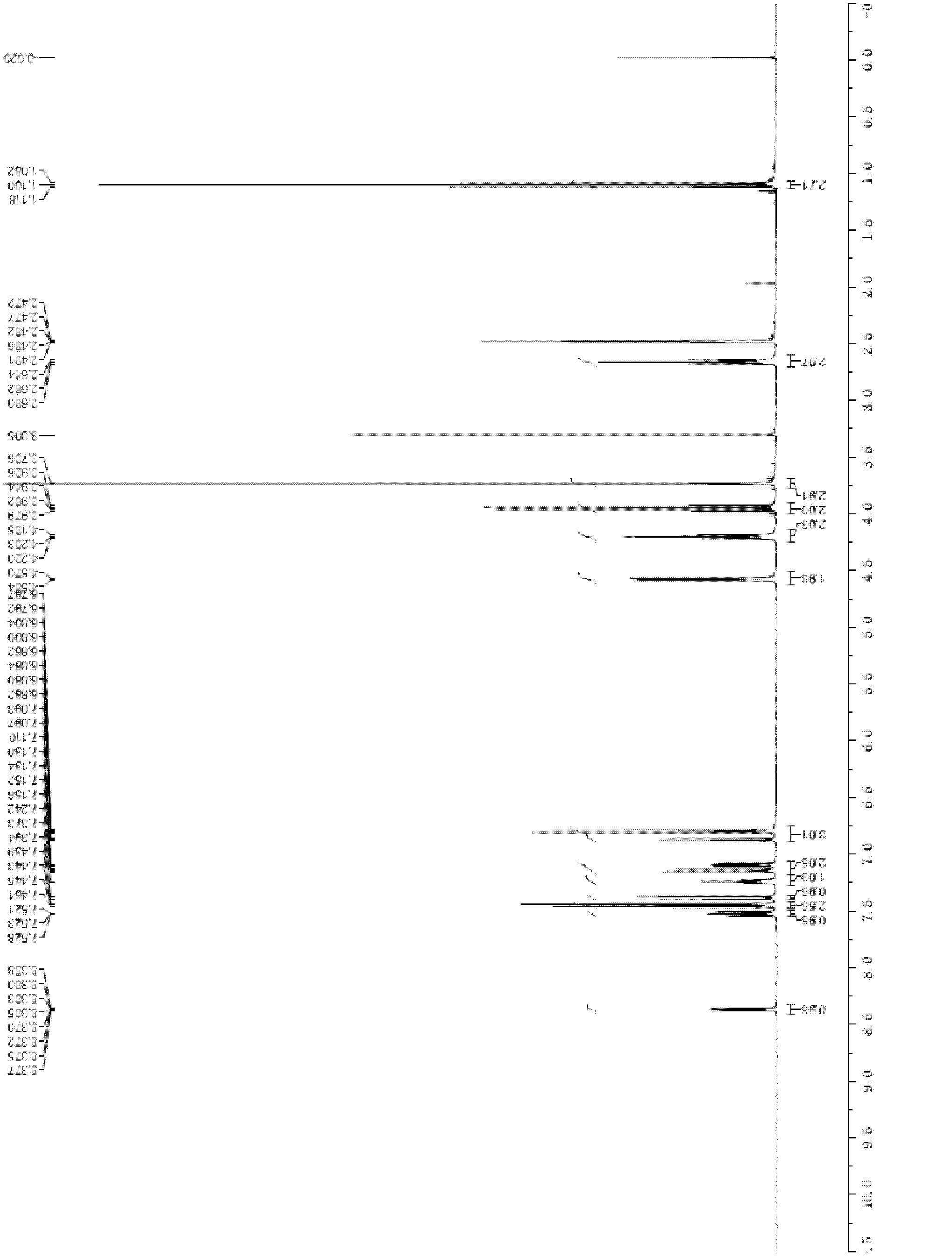
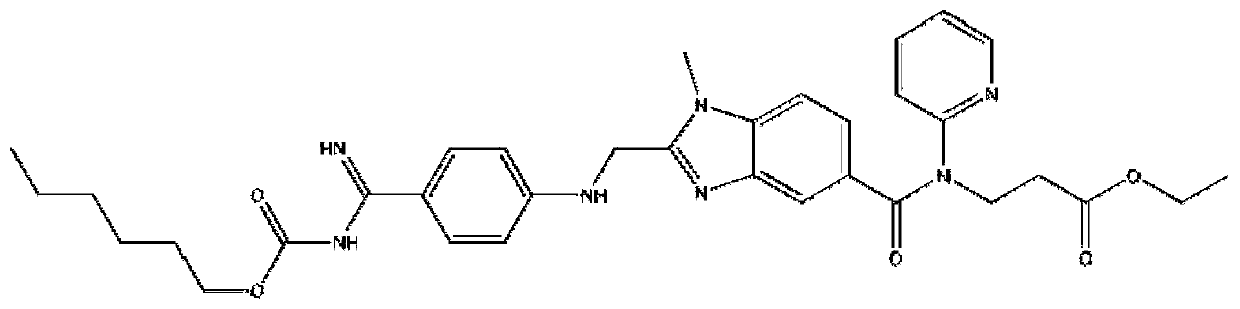
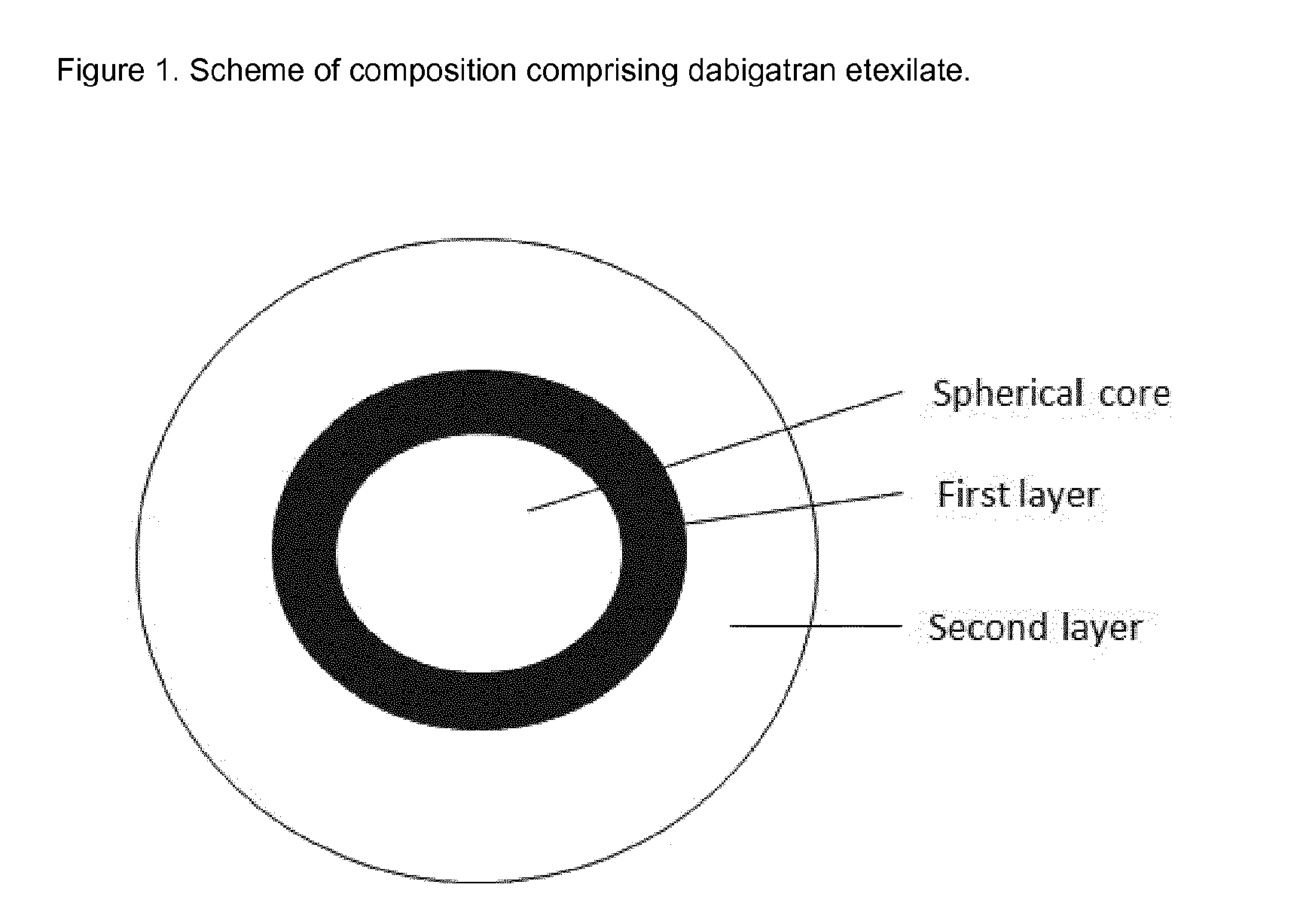
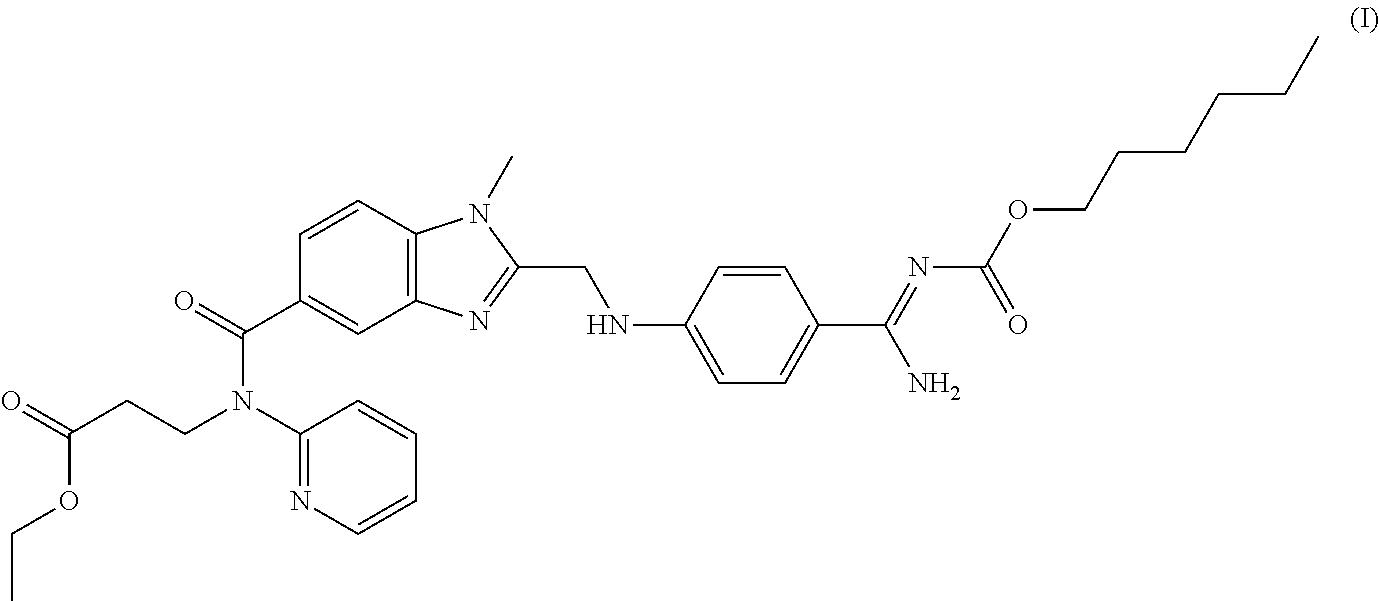
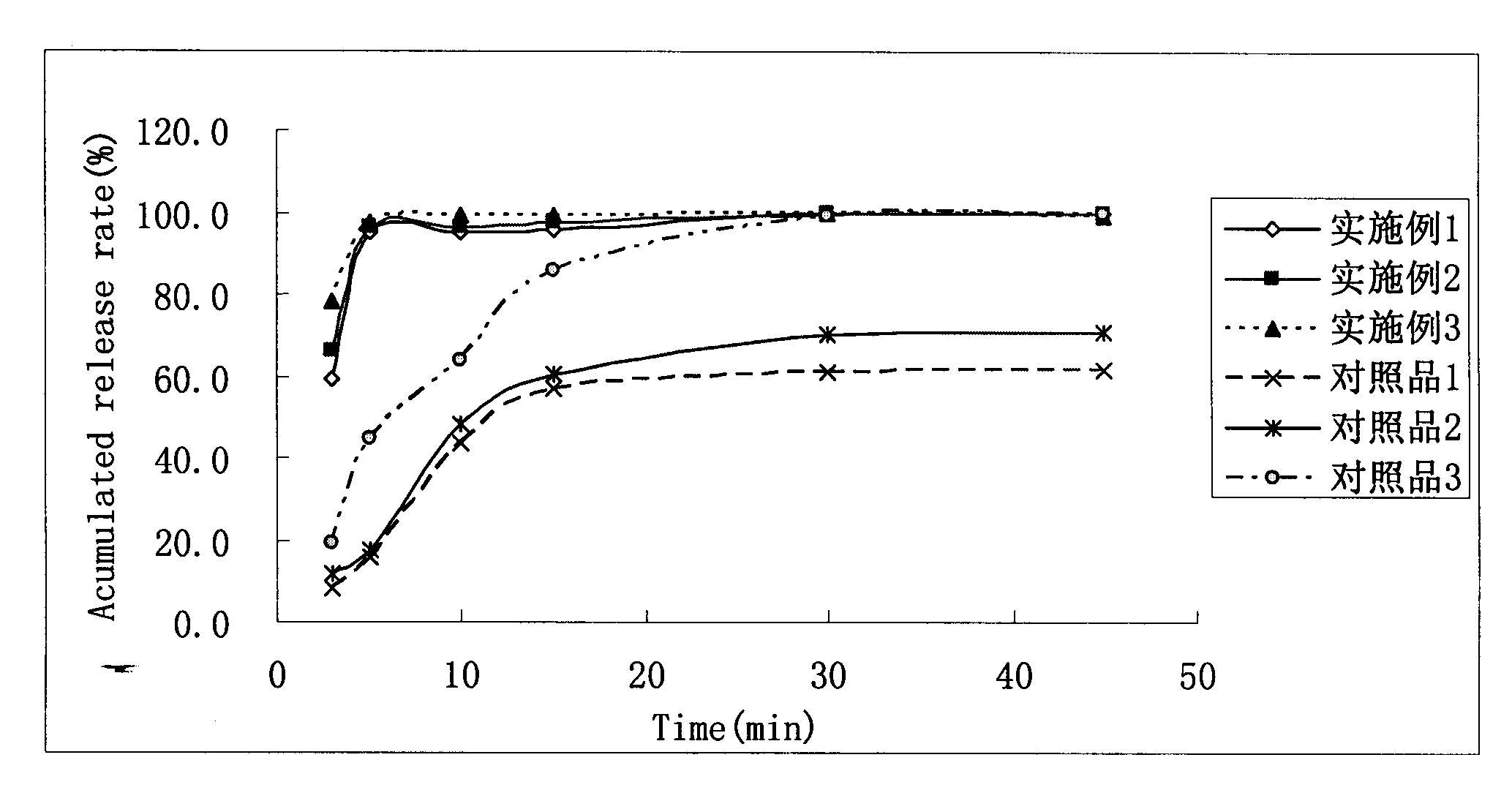
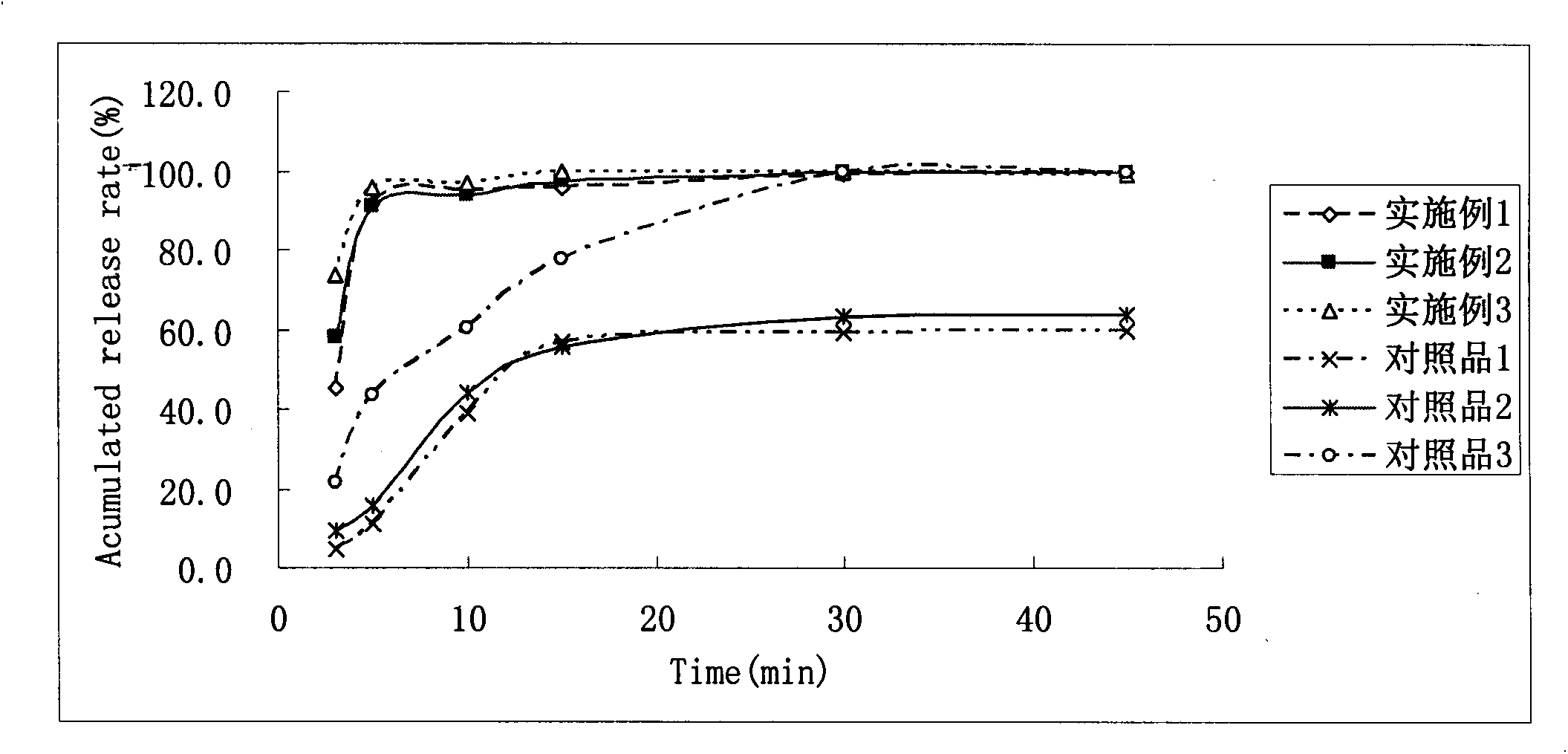
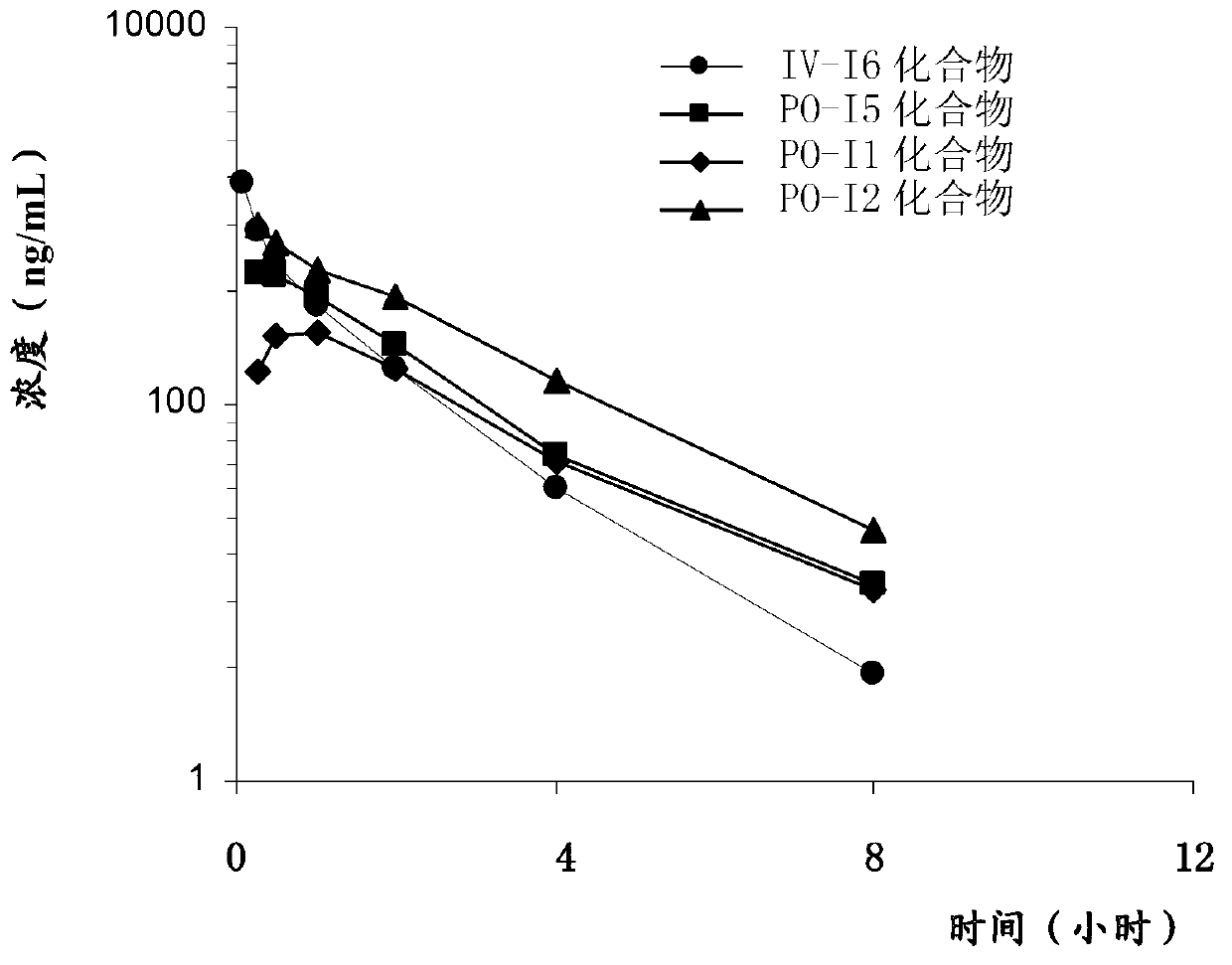
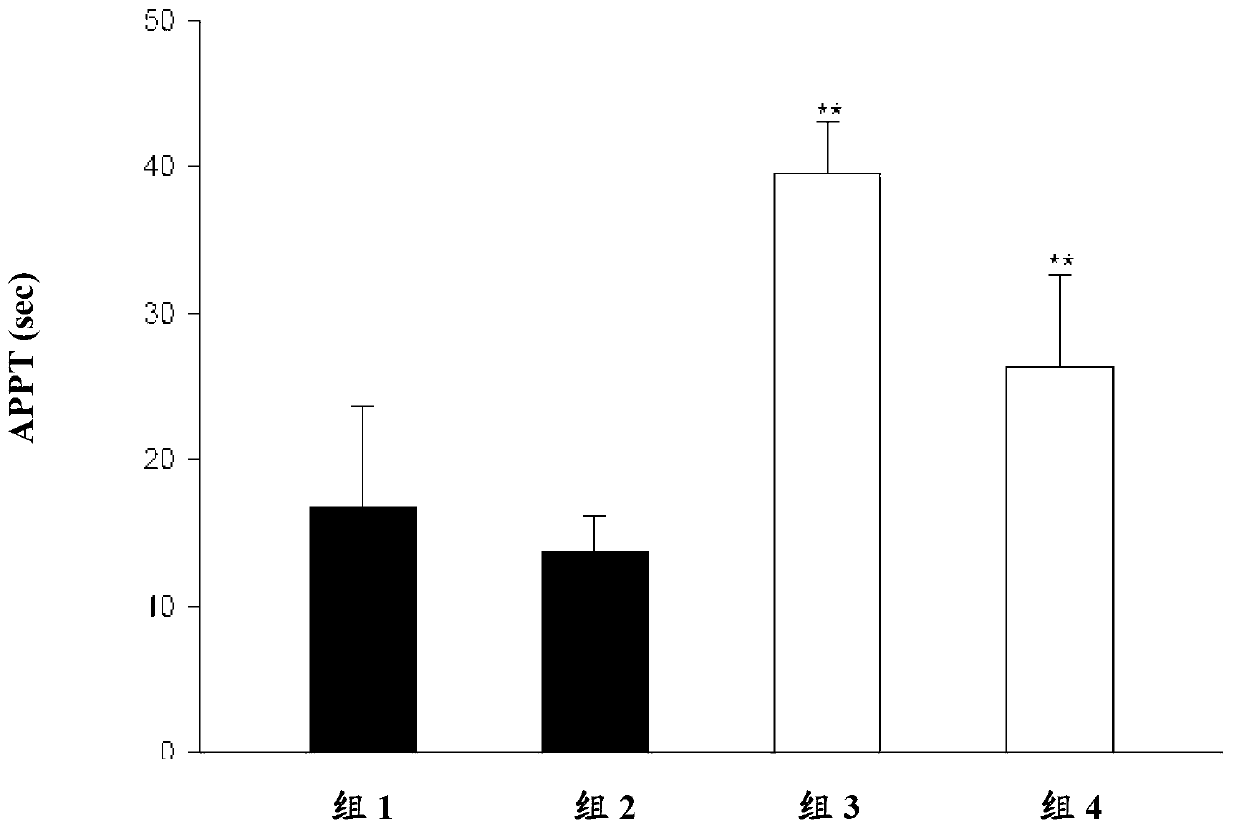
Patents
Literature
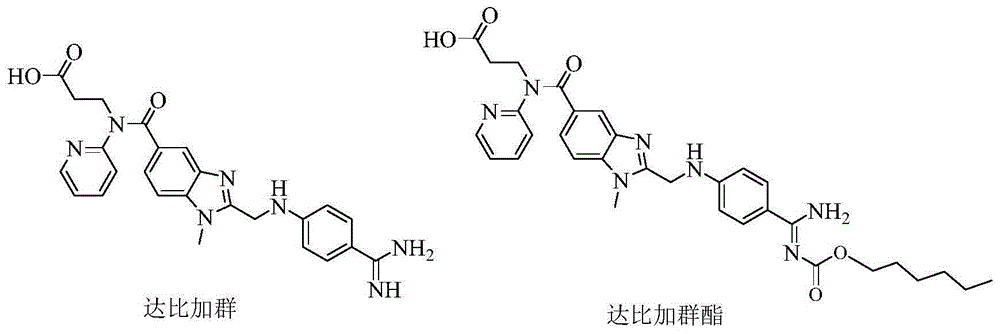
175 results about "Dabigatran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
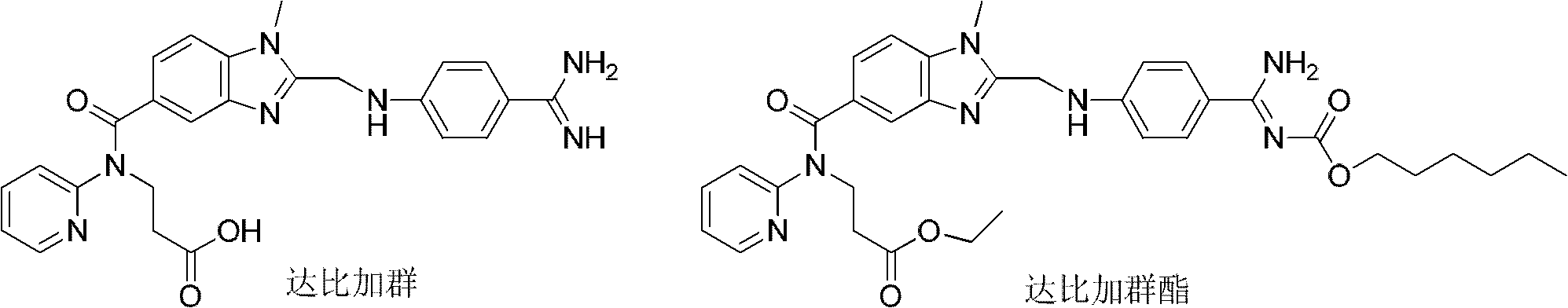
Dabigatran is used to prevent stroke and harmful blood clots (such as in your legs or lungs) if you have a certain type of irregular heartbeat (atrial fibrillation). Dabigatran is also used to treat blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism) and to reduce the risk of them occurring again. This medication may also be used to prevent these blood clots from forming after hip replacement surgery.
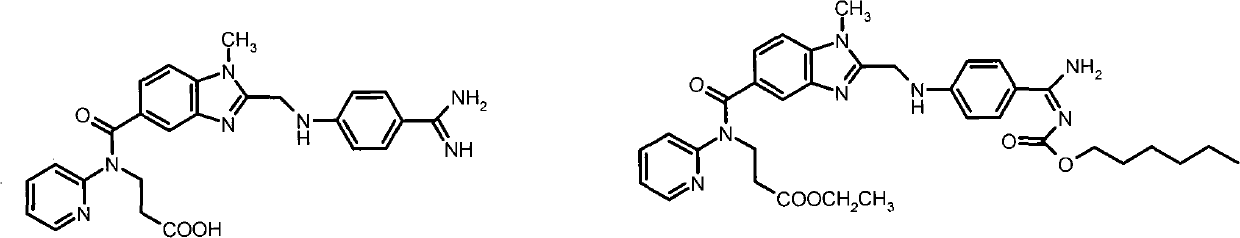
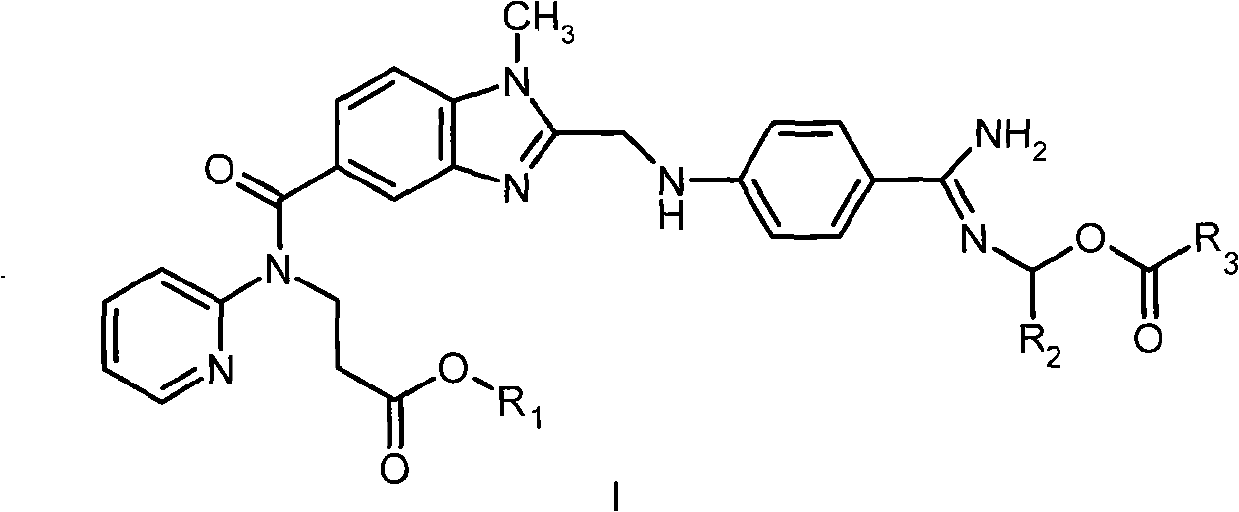
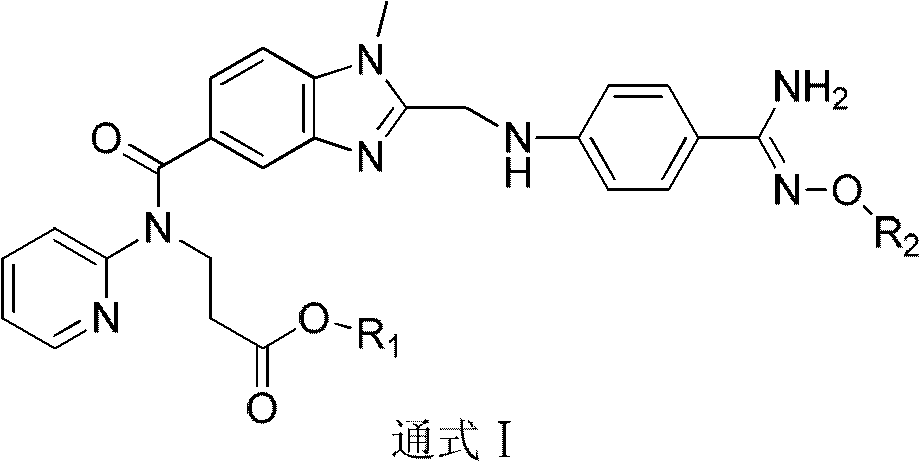
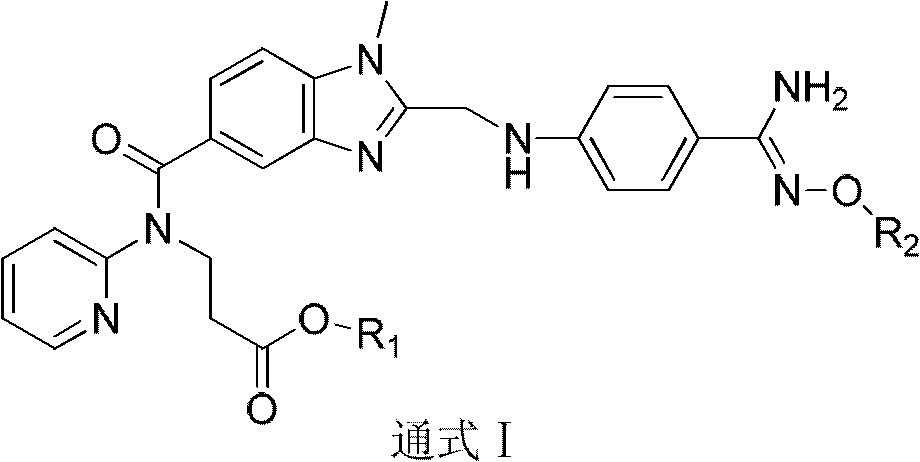
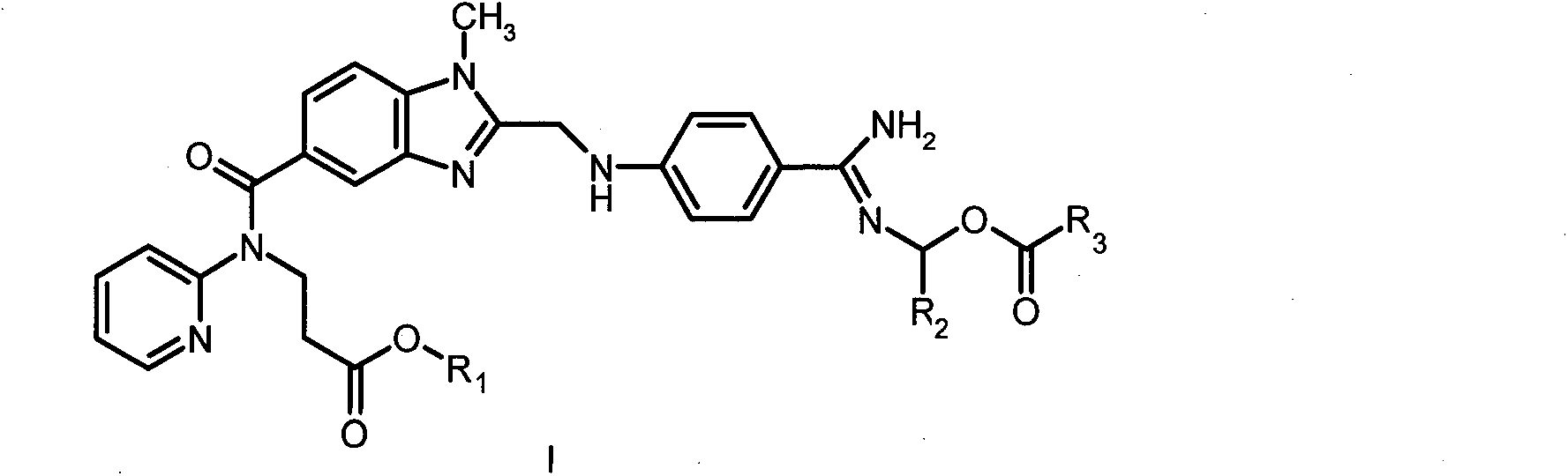
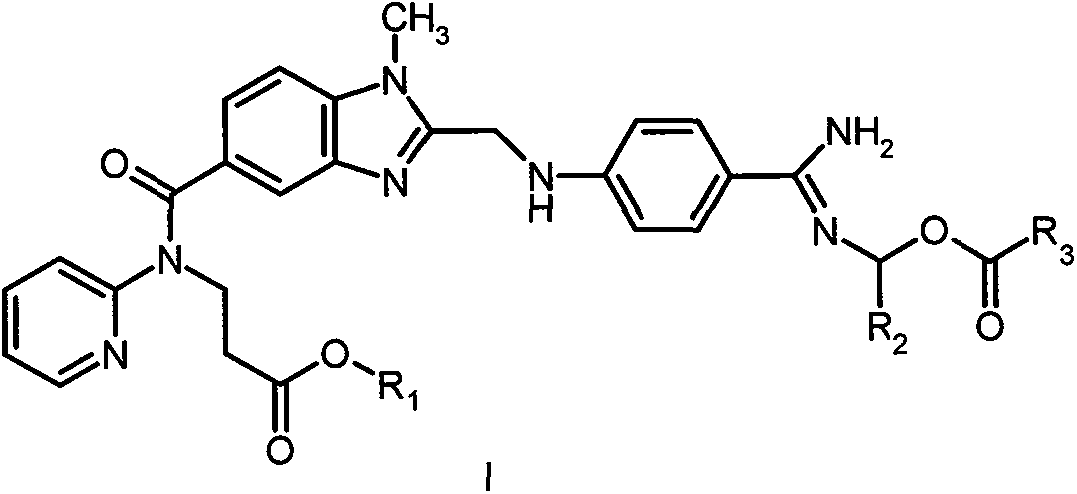
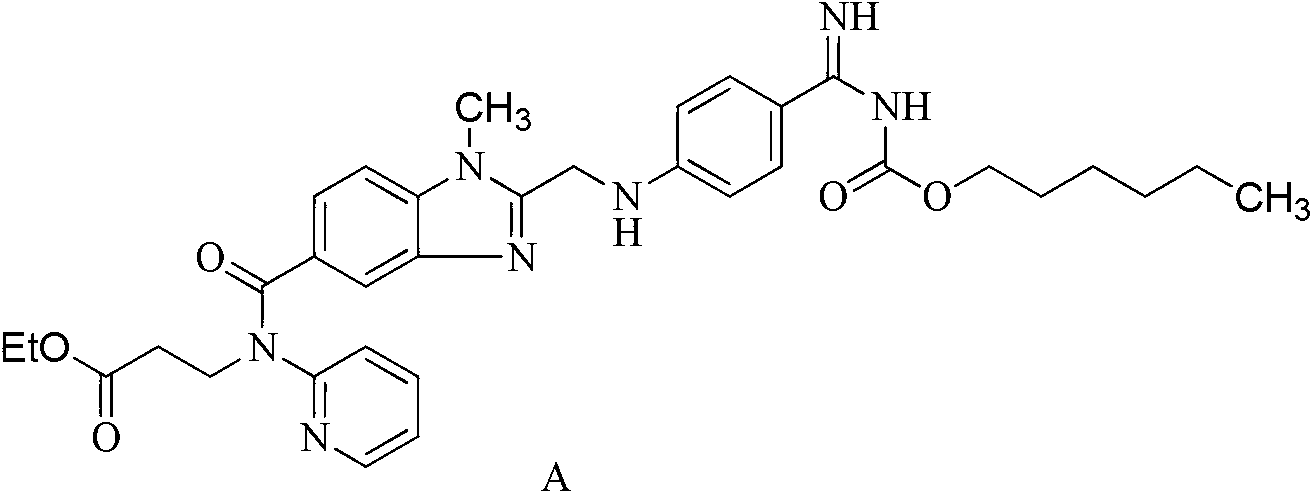
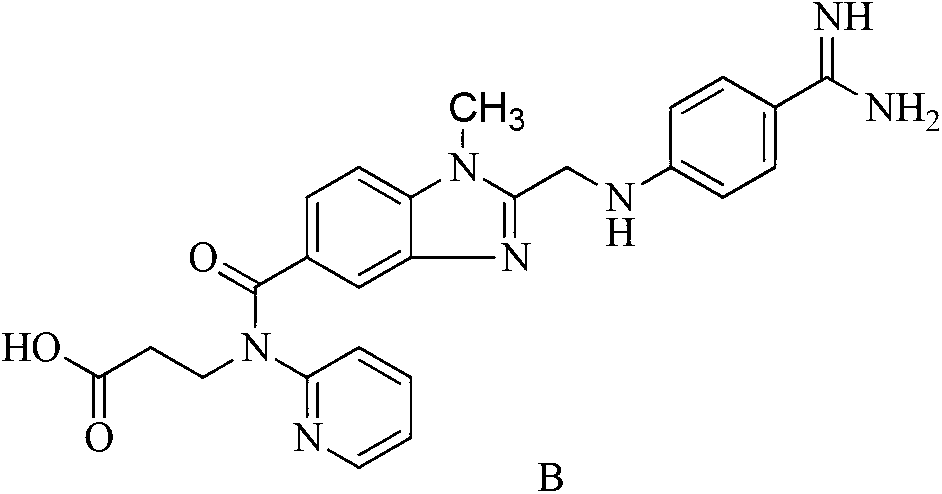
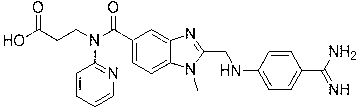
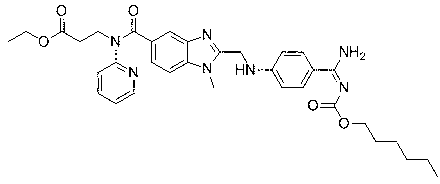
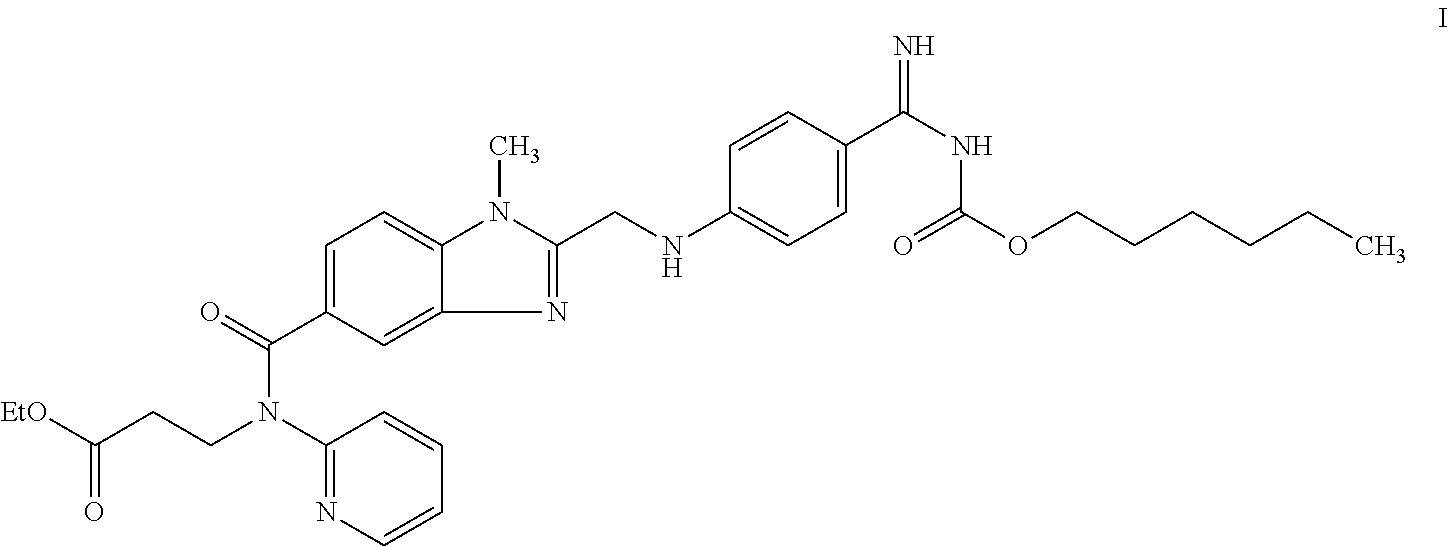
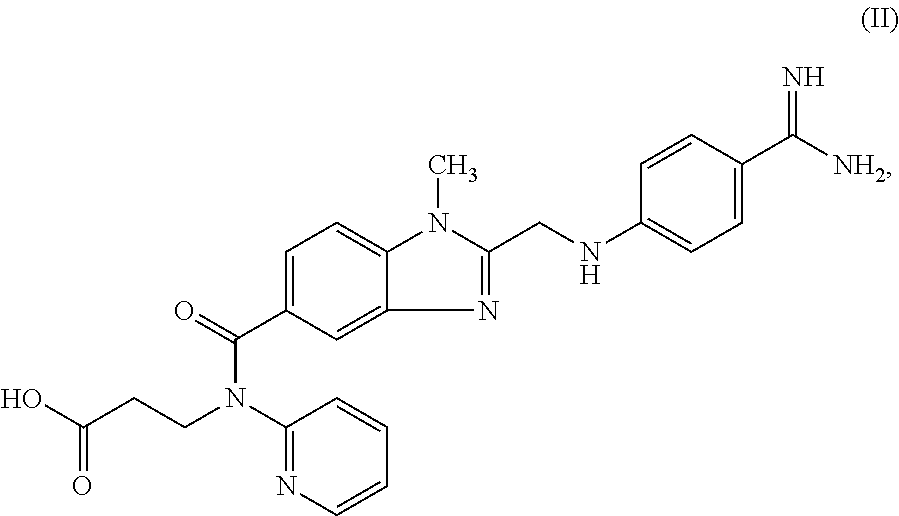
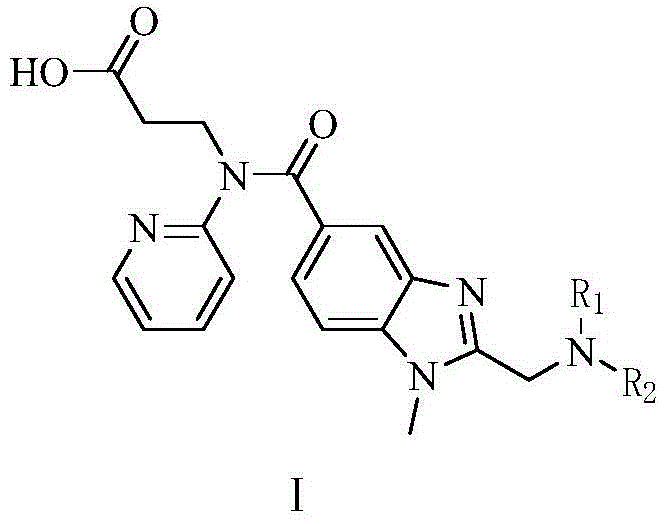
Dabigatran ester derivatives as prodrug
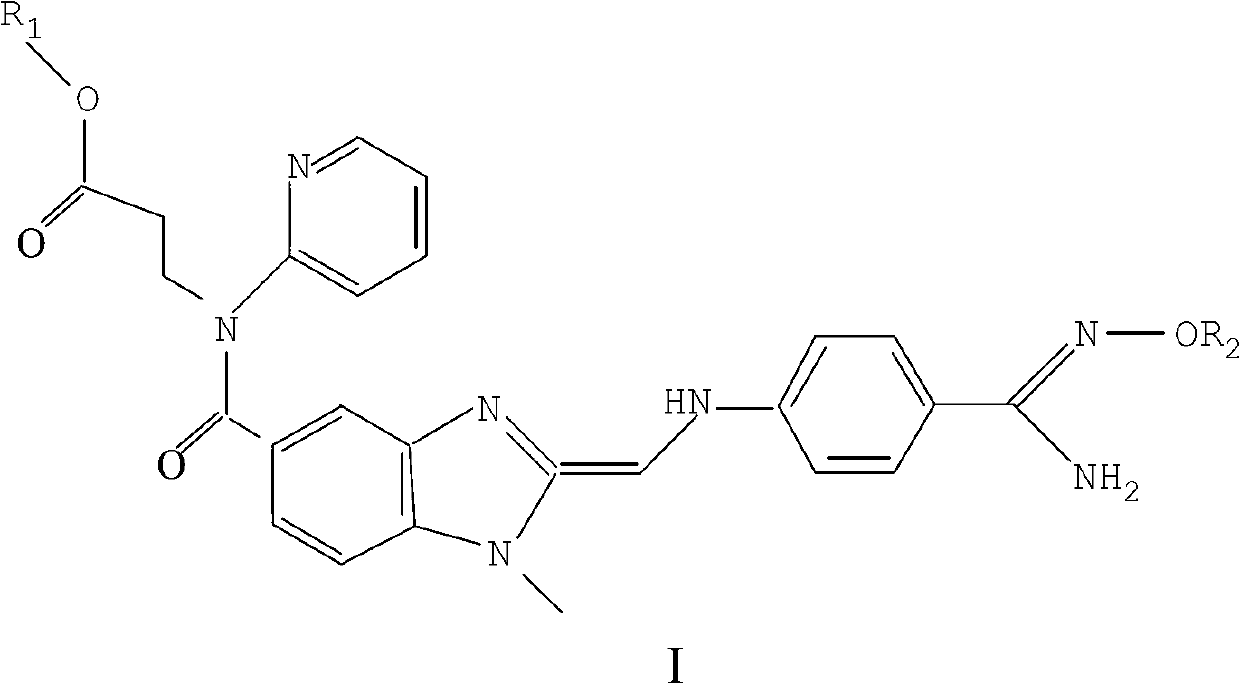
The invention relates to dabigatran ester derivatives as shown in a constitutional formula I, pharmaceutically acceptable nontoxic salts thereof, medical composite containing the compounds as active components, and application of thrombin inhibitor of the compounds and medical composites, wherein R1 represents H or C1 to C5 alkyl; R2 represents H, or C1 to C3 alkyl; and R3 represents C1 to C8 alkyl or substituted alkyl.
Owner:北京弘生医药科技有限公司
Dabigatran etexilate intermediate, preparation method for same and method for preparing dabigatran etexilate
InactiveCN102633713AHigh product yieldHigh product purityOrganic chemistryReaction temperatureStructural formula
The invention relates to a dabigatran etexilate intermediate, a preparation method for the same and a method for preparing dabigatran etexilate, which belong to the technical field of pharmaceutical chemistry and pharmaceutical engineering. The dabigatran etexilate intermediate is represented as a structural formula (2), so that a preparation process for the dabigatran etexilate is simplified, yield and purity of the dabigatran etexilate are high, reaction temperature is low, expensive dehydrating agent is not needed, and good market prospect is provided.
Owner:NANJING UNIV OF TECH +1
Pharmaceutical composition containing dabigatran etexilate or salt and hydrate thereof
ActiveCN103127109AImprove solubilityHigh dissolution rateOrganic active ingredientsCapsule deliverySolubilityDissolution
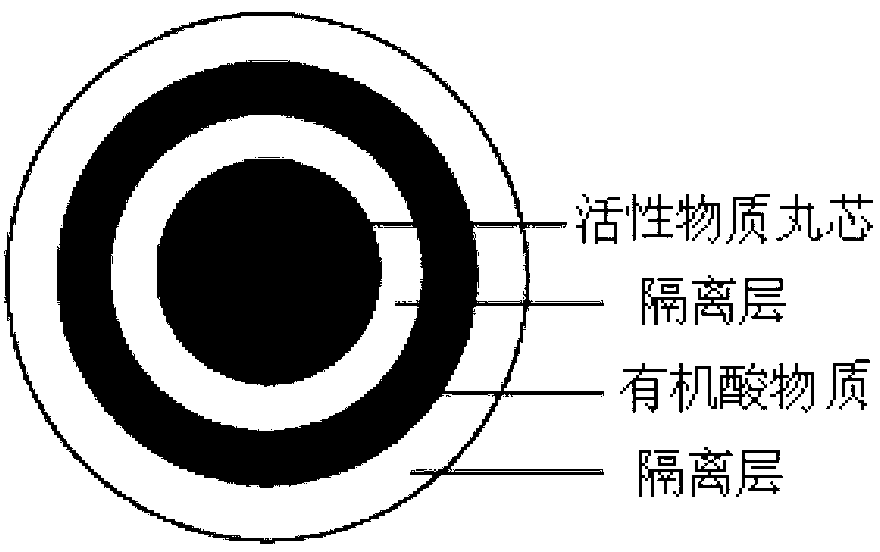
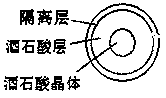
The invention discloses a pharmaceutical composition containing dabigatran etexilate or salt and hydrate of the dabigatran etexilate. The pharmaceutical composition comprises an active substance pill core material containing the dabigatran etexilate or its pharmaceutically acceptable salt or hydrate and a filling agent and / or a bonding agent,and an organic acid or an organic acid material layer, the organic acid is arranged outside the active substance pill core materials, the organic acid material layer contains the organic acid, and the organic acid is selected from tartaric acid, succinic acid, citric acid, glutamic acid, fumaric acid, malic acid, aspartic acid or their hydrates or salts. After screening, the dabigatran etexilate active substance is prepared into a pill core, the organic acid material layer is added in an integrated mode, and meanwhile, an isolated layer is applied between the dabigatran etexilate active substance pill core and the organic acid material layer. The prepared dabigatran etexilate oral drugs composition has the advantages that solubility and dissolution rate are good, and bioavailability effect is prominent.
Owner:NANJING HUAWE MEDICINE TECH DEV
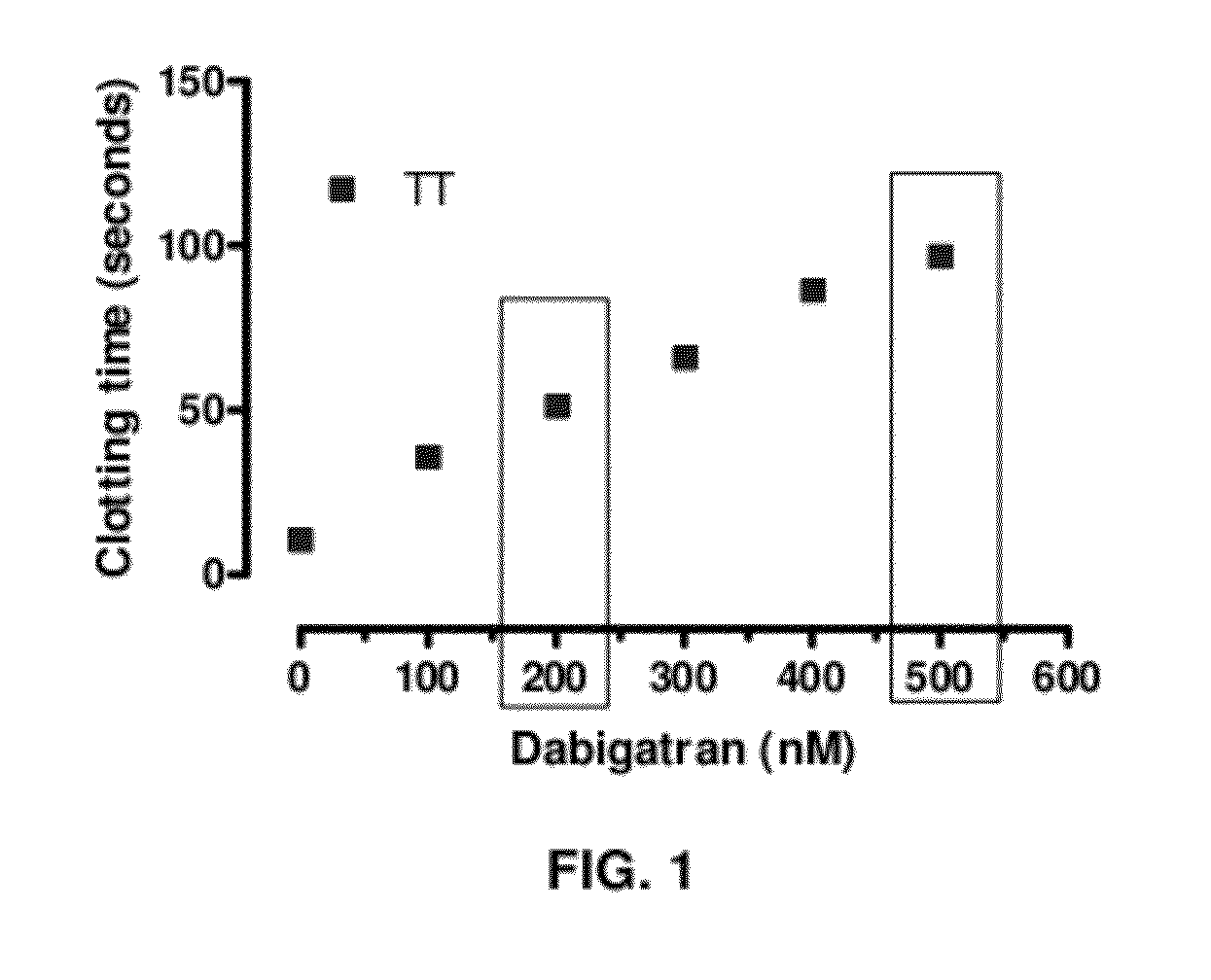
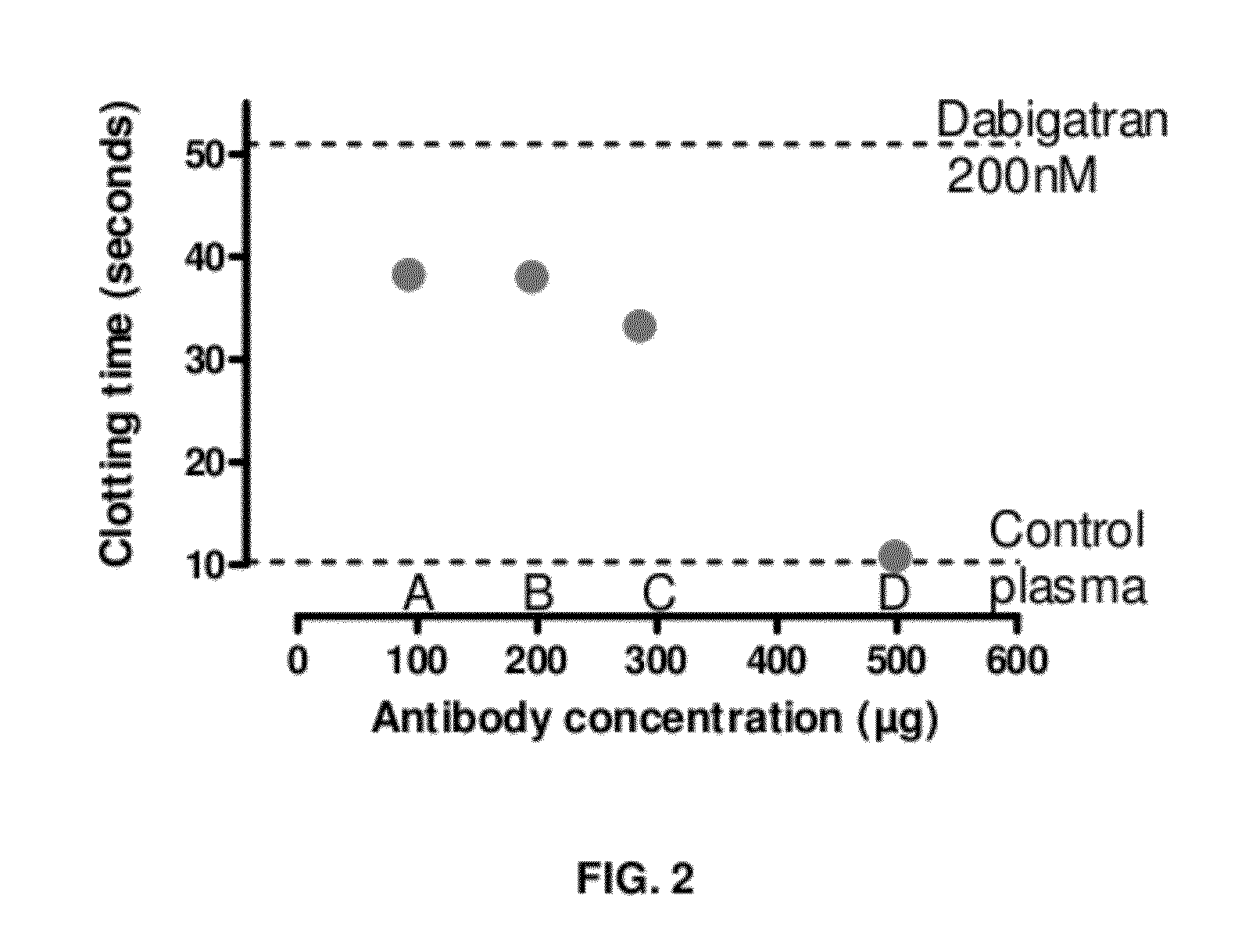
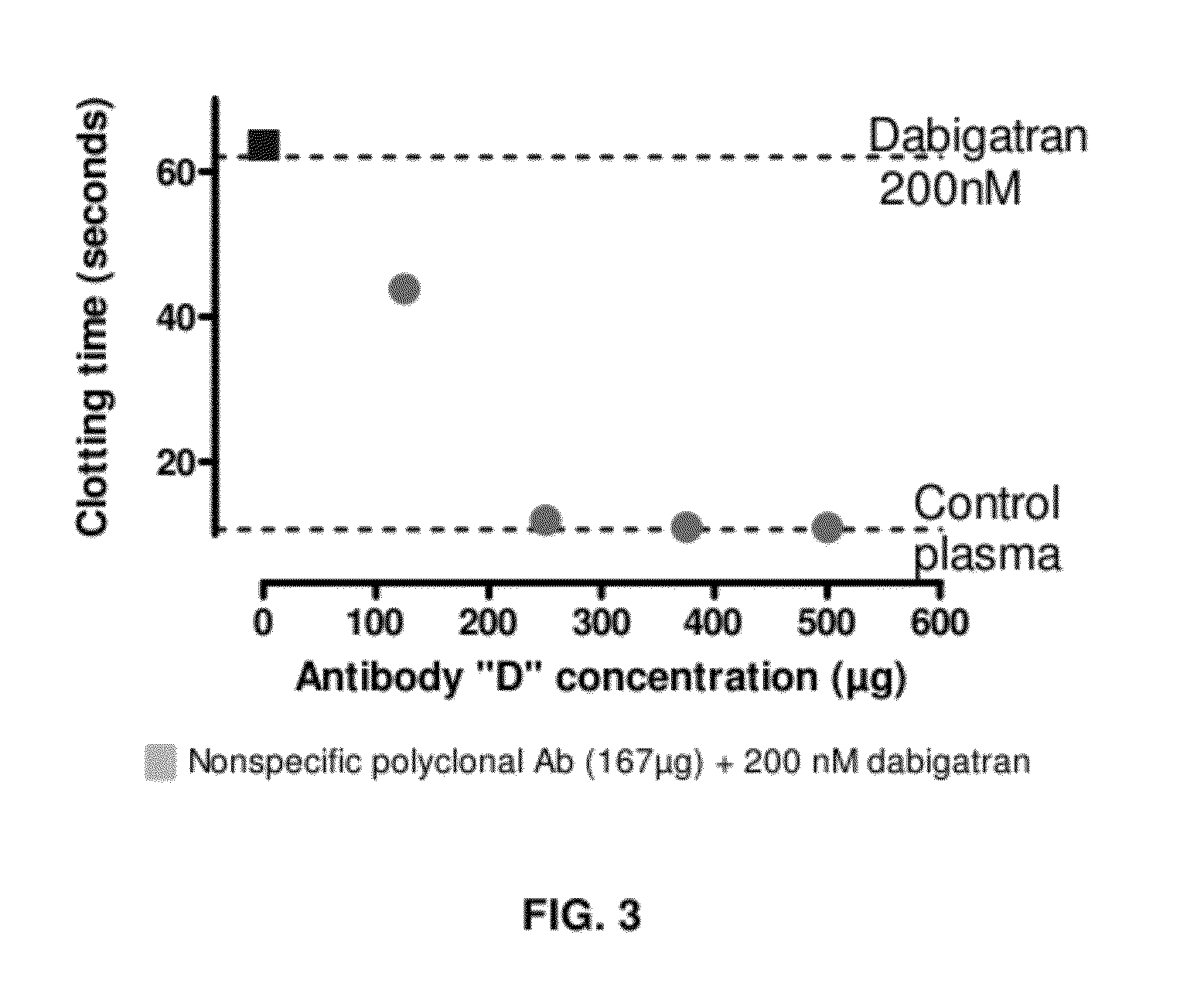
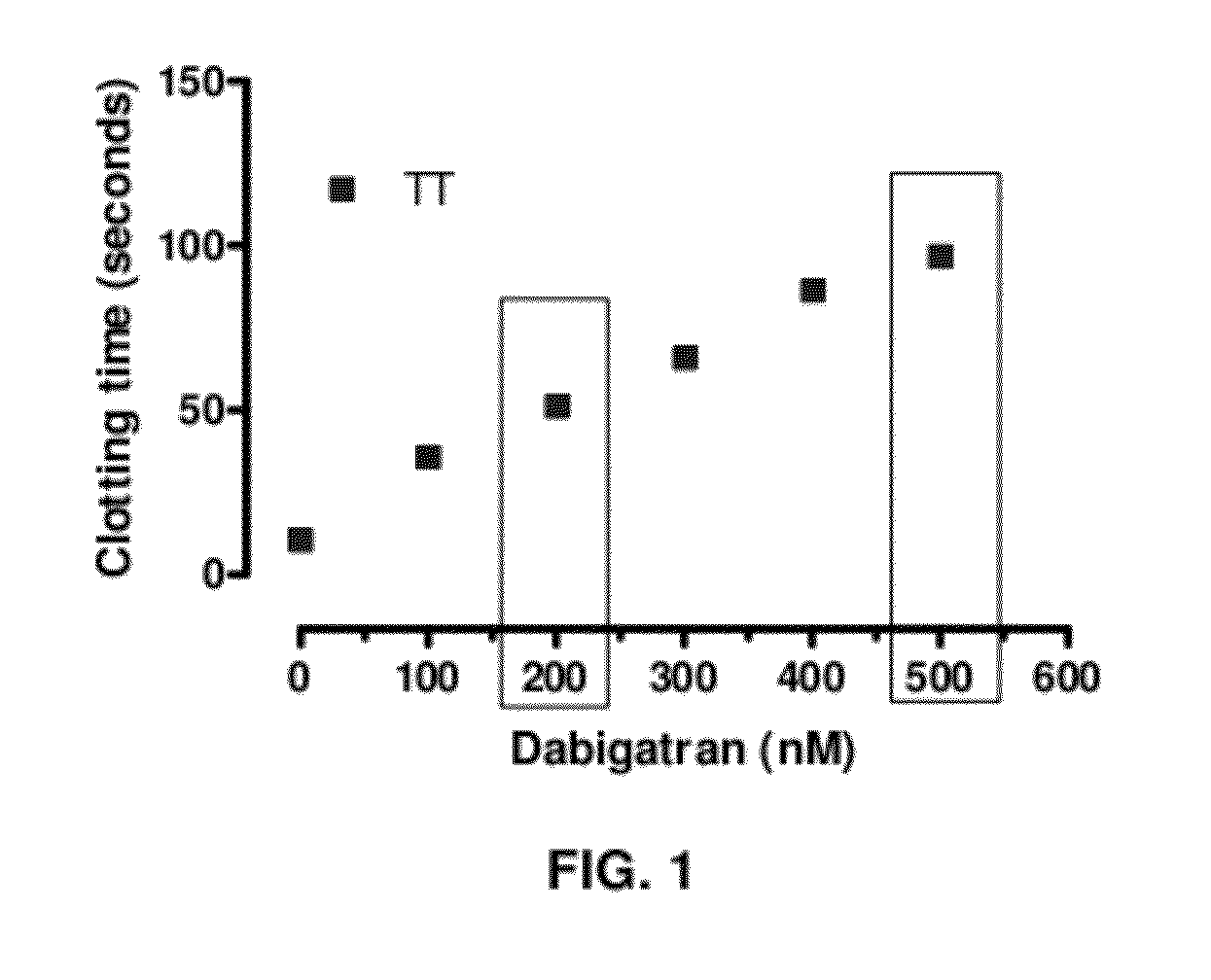
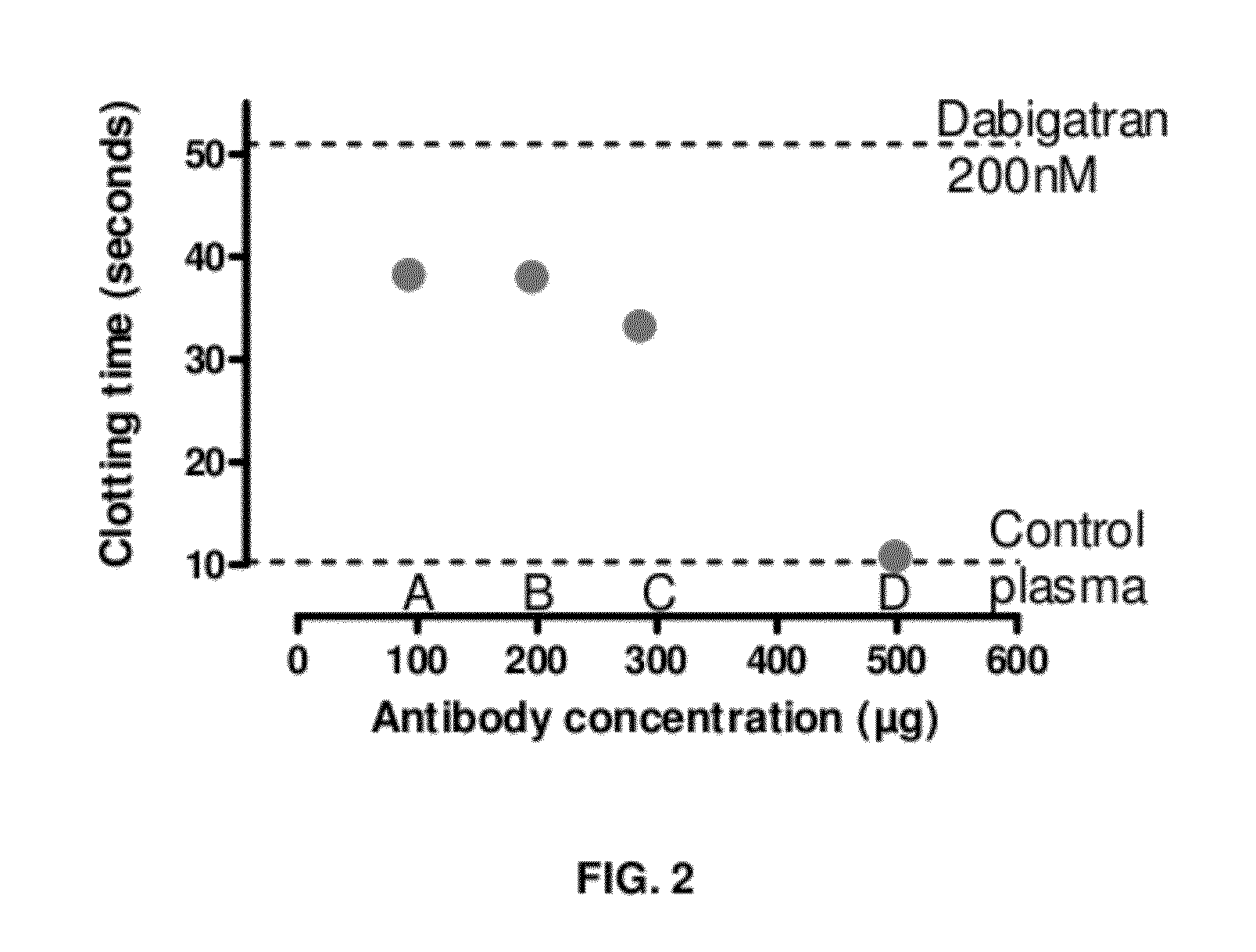
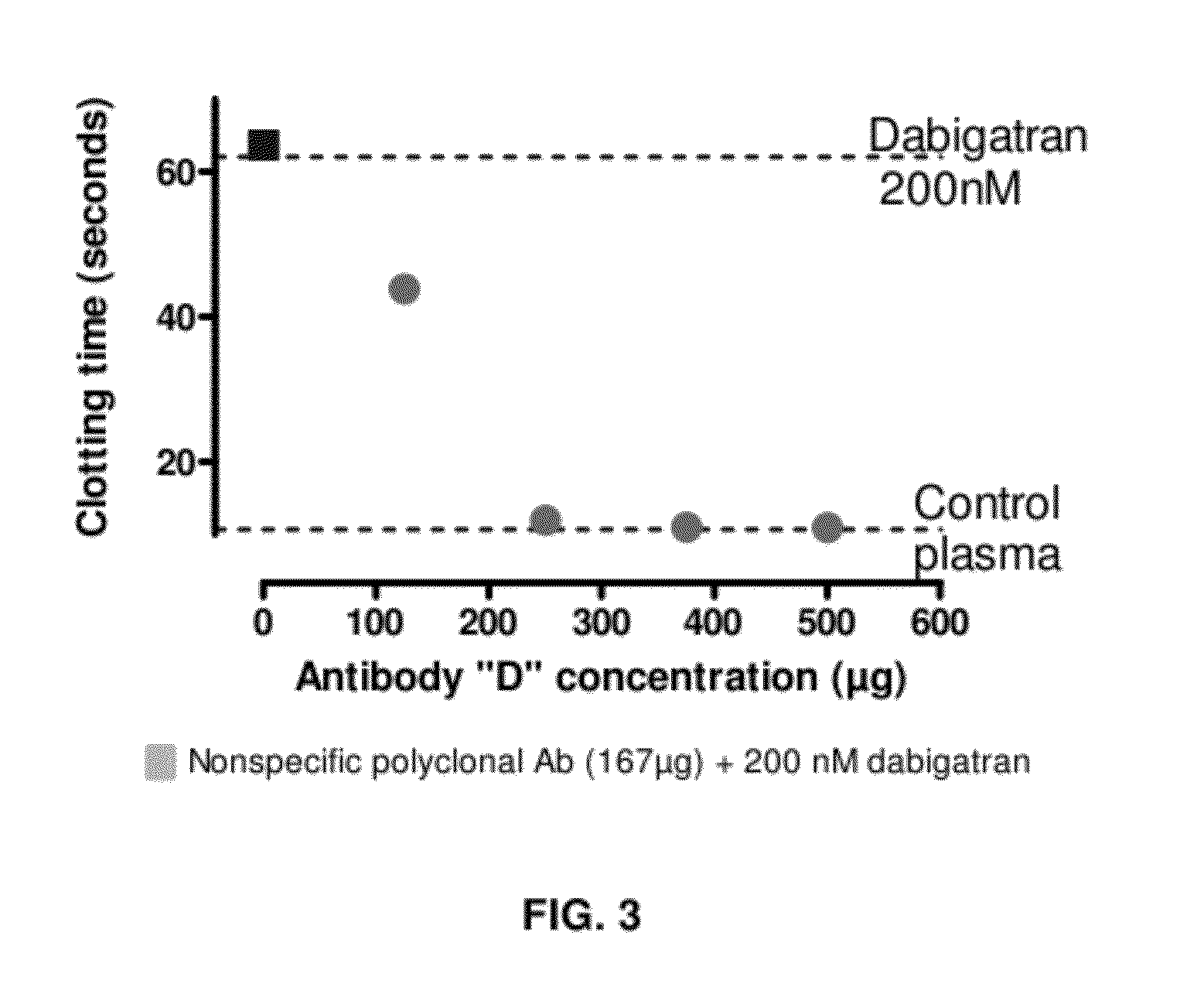
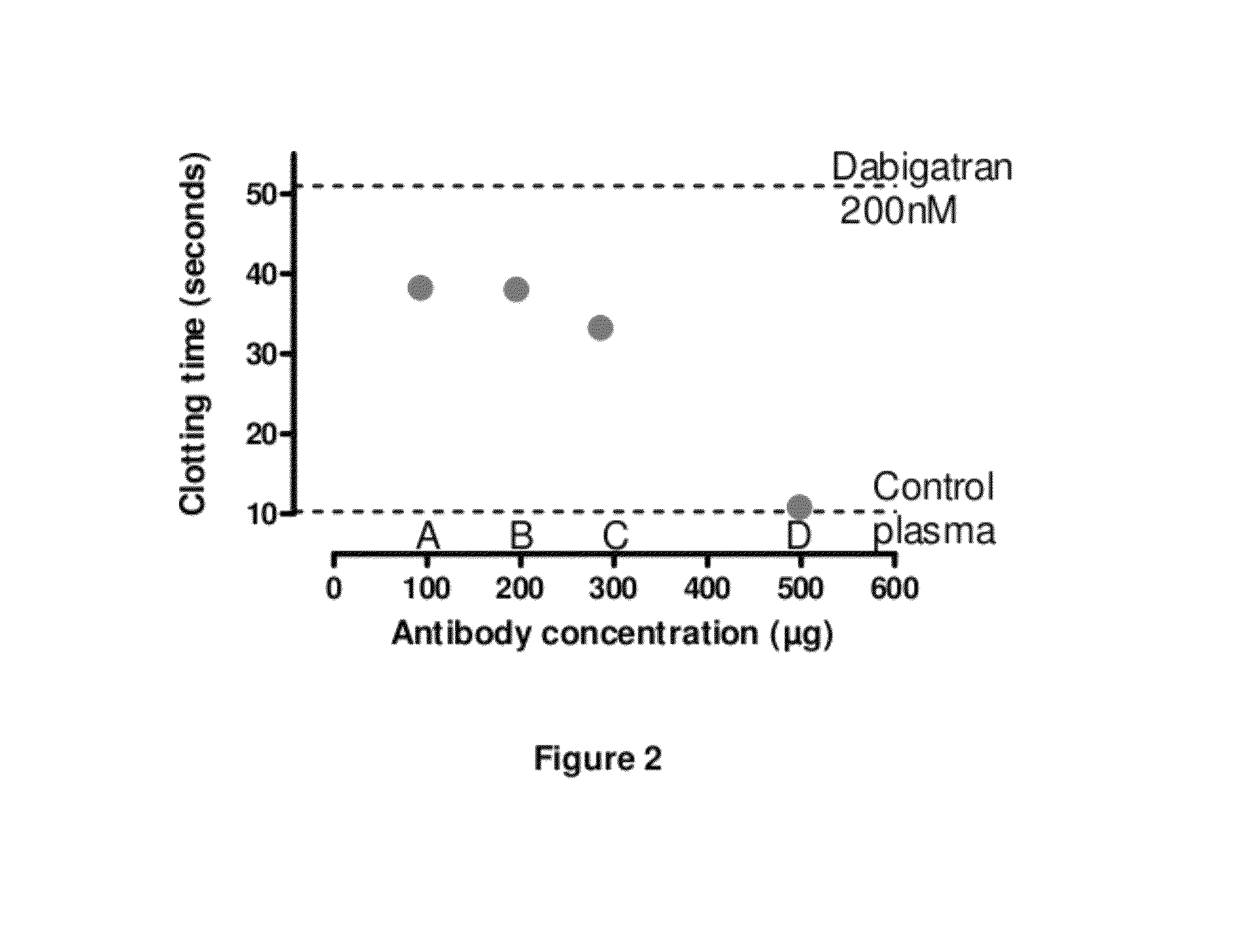
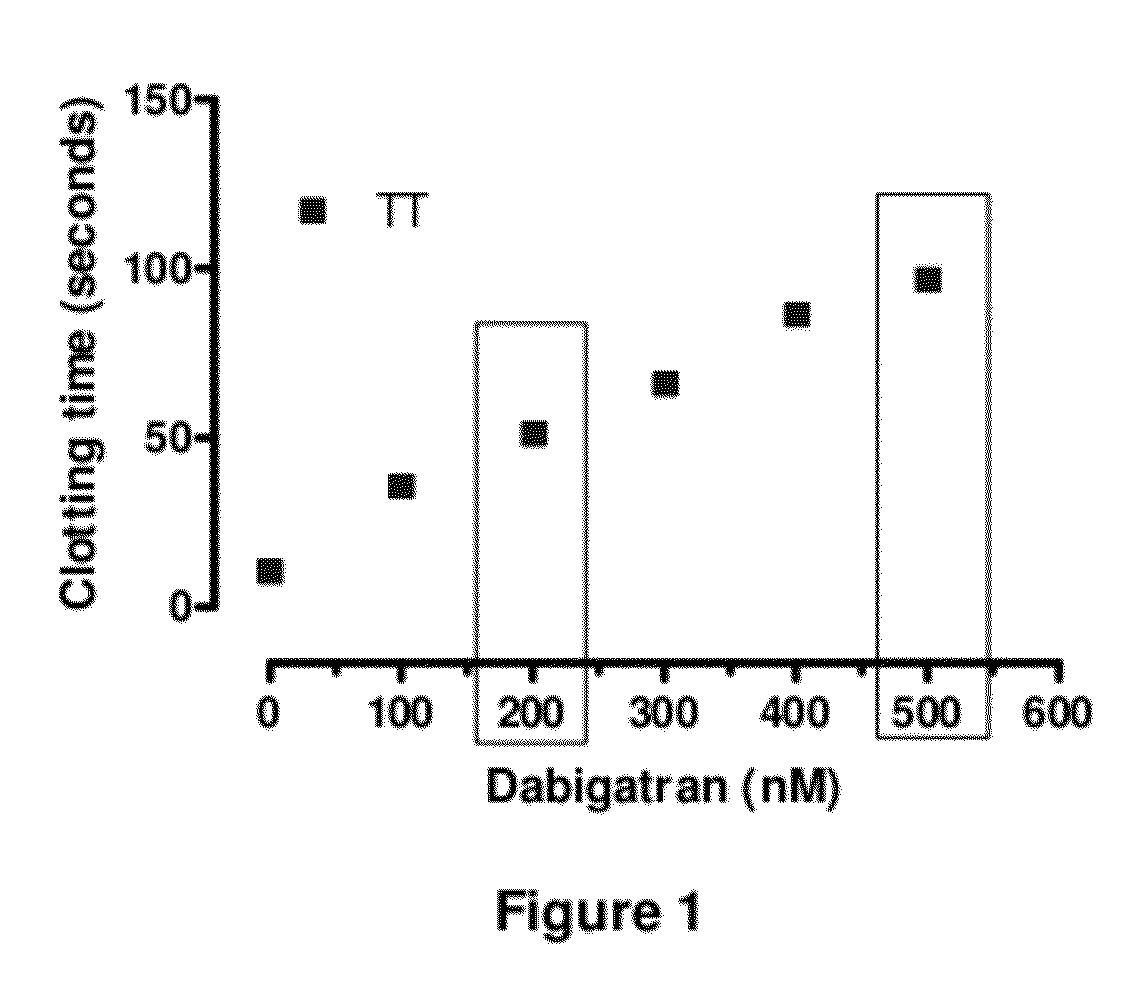
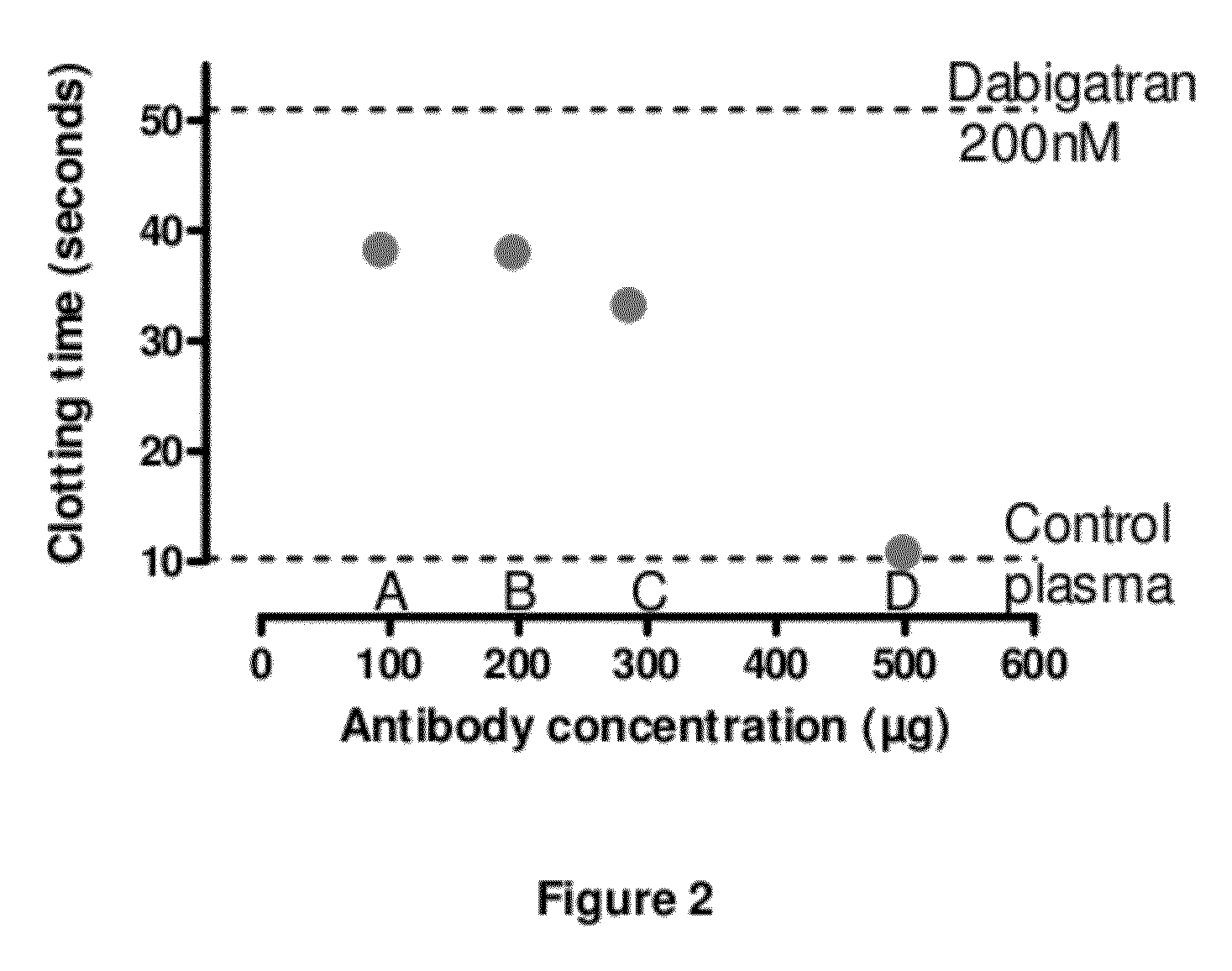
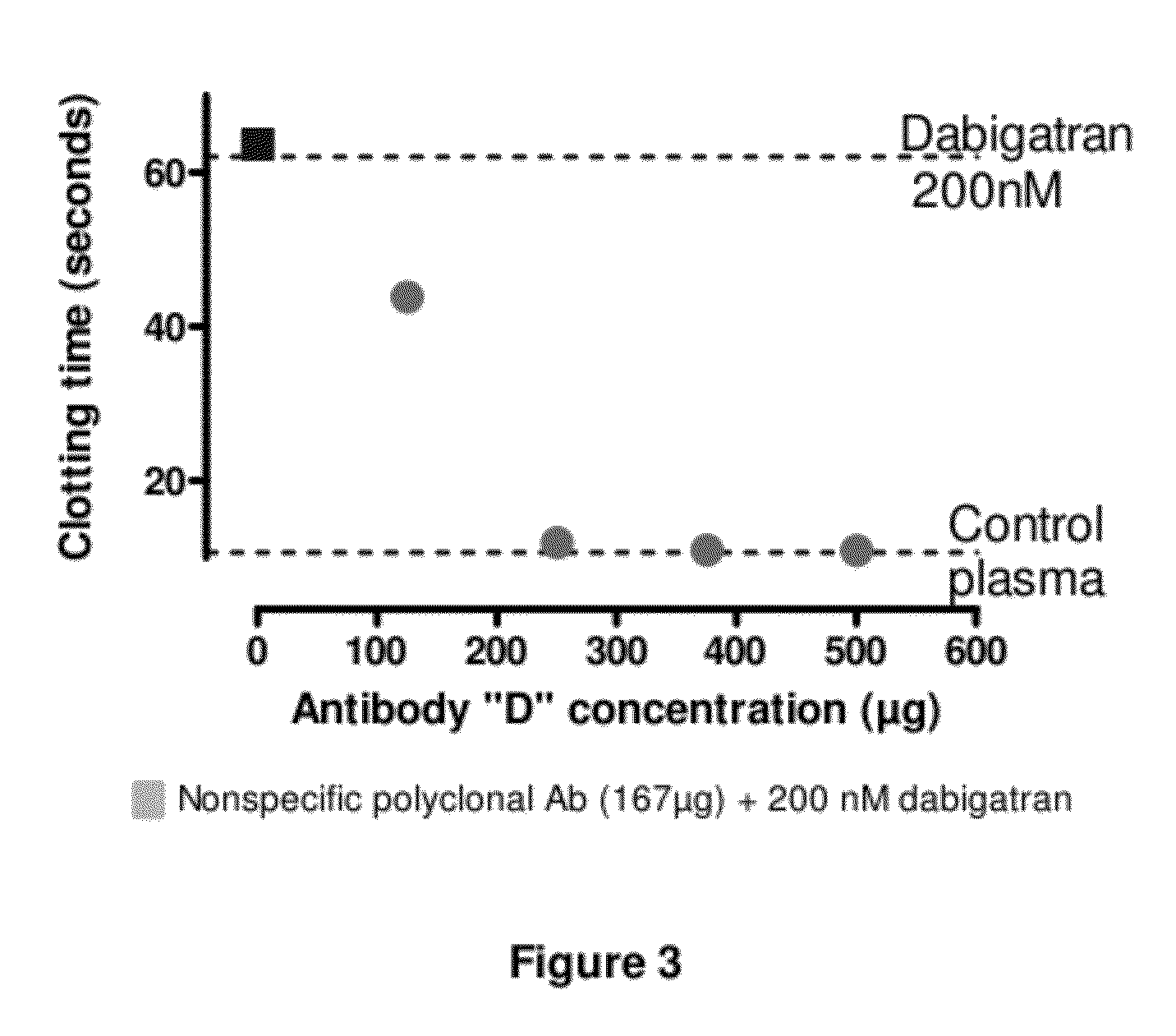
Anticoagulant antidotes comprising antibodies that bind dabigatran and/or related compounds
The present invention relates to antibody molecules against anticoagulants, in particular dabigatran, and their use as antidotes of such anticoagulants.
Owner:BOEHRINGER INGELHEIM INT GMBH
A dabigatran etexilate solid dispersion enteric-coated preparation and a preparing method thereof
InactiveCN106880845AInhibition of re-coalescenceHigh dissolution rateOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPartition coefficient
A dabigatran etexilate enteric-coated preparation and a preparing method thereof are provided. Dabigatran etexilate that is a medicine active component is prepared into a solid dispersion through a hot melting extrusion technique, and further prepared into the enteric-coated preparation. The preparation overcomes dependence of solubility and oil-water partition coefficients of the dabigatran etexilate and salts thereof on pH values, improves membrane permeability of a medicine in intestinal juice, improves medicine absorption, avoids or reduces hepatic first-pass effects, increases medicine bioavailability, and avoids hydrolysis of water-insoluble dabigatran etexilate and adverse irritation of the medicine on the gastrointestinal tract. A process is simple. Medicine properties are stable. The preparation is convenient to take.
Owner:GUIZHOU YIBAI PHARMA CO LTD
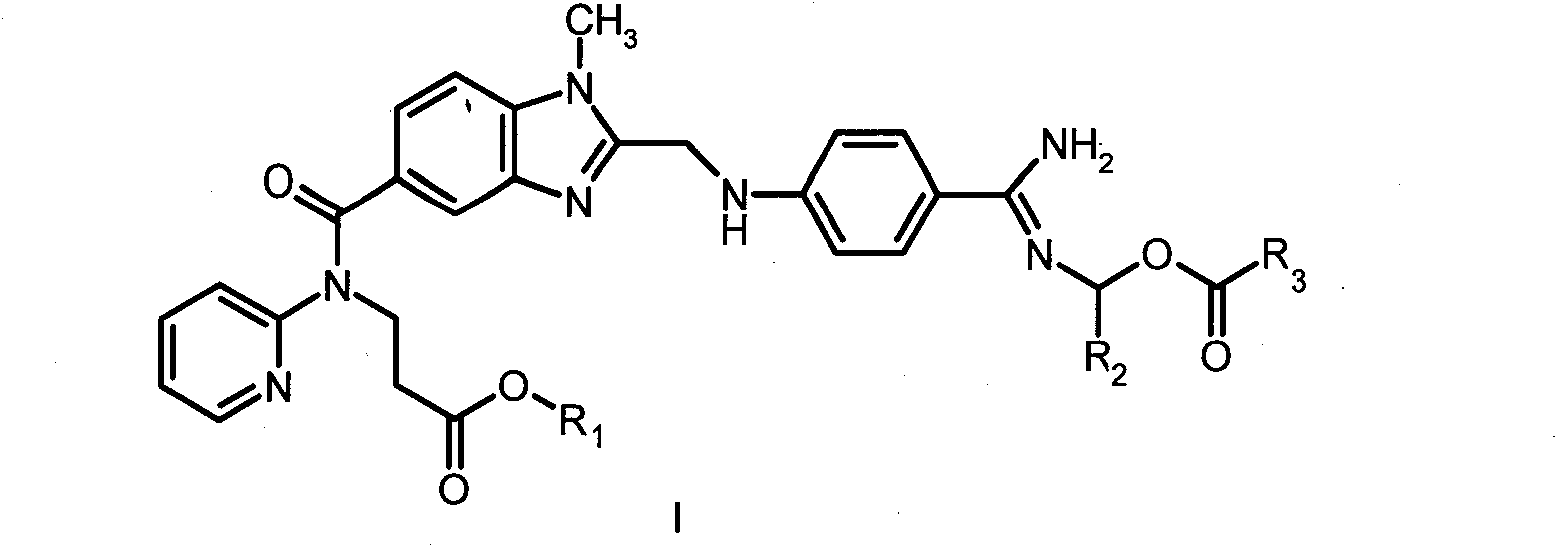
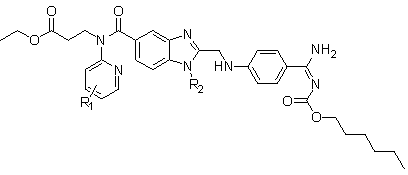
Dabigatran derivatives and preparation method thereof
InactiveCN102875529AOrganic active ingredientsOrganic chemistryBenzimidazole derivativeThrombin activity
The invention relates to Dabigatran derivatives and pharmaceutically acceptable salts thereof, which are shown in a general formula I, a preparation method for the derivatives in the formula I, medical compositions comprising the compounds as active ingredients, and use of the compounds and the medical compositions as thrombin inhibitors. In the formula I, R1 and R2 are respectively defined in the specifications.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
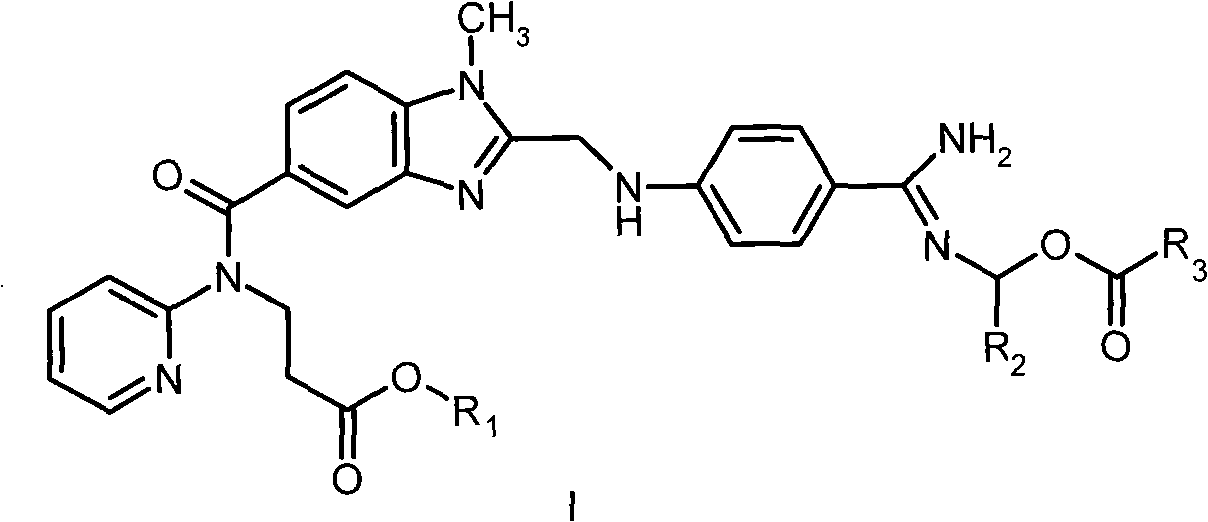
Dabigatran ester derivatives as prodrug
The invention relates to dabigatran ester derivatives as shown in a constitutional formula I, pharmaceutically acceptable nontoxic salts thereof, medical composite containing the compounds as active components, and application of thrombin inhibitor of the compounds and medical composites, wherein R1 represents H or C1 to C5 alkyl; R2 represents H, or C1 to C3 alkyl; and R3 represents C1 to C8 alkyl or substituted alkyl.
Owner:北京弘生医药科技有限公司
Novel salts for the manufacture of pharmaceutical compositions
InactiveCN102858762AMorphologically uniformOrganic active ingredientsOrganic chemistryCombinatorial chemistryDabigatran ethyl ester
The present invention relates to novel polymorphous salts of dabigatran etexilate of the formula 11 and process for the preparation thereof.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENYTARSASAG (EGIS PHARMA PLC)
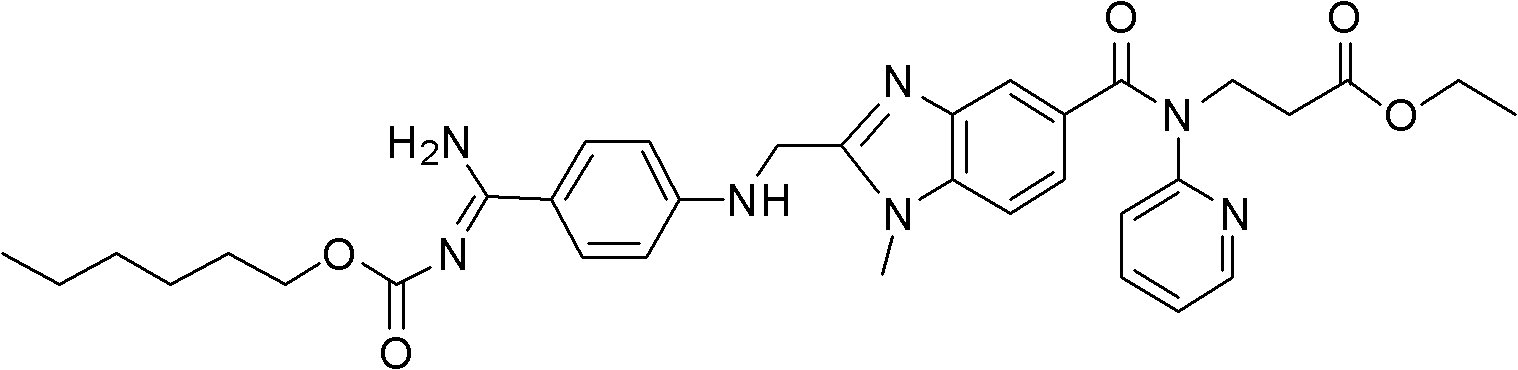
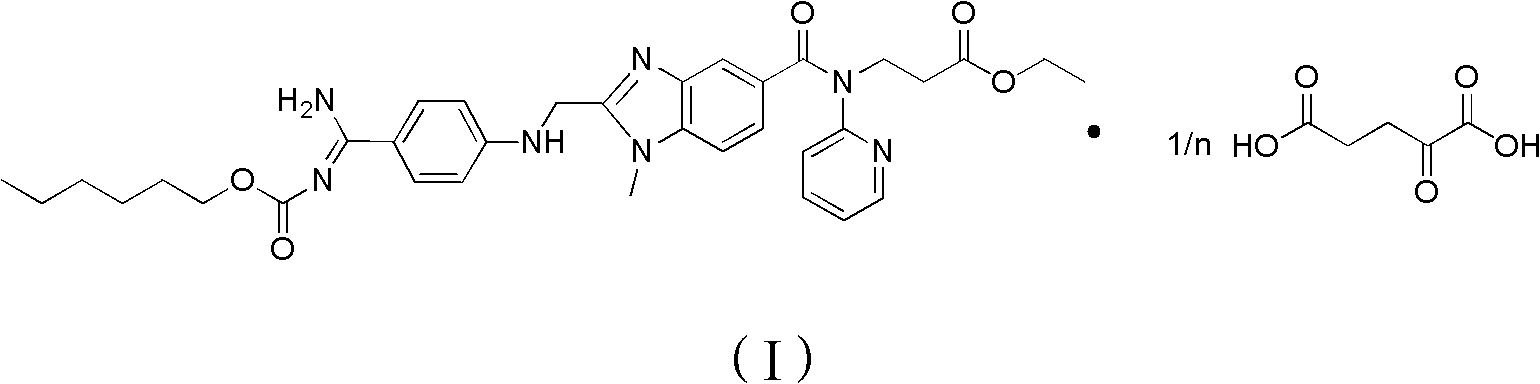
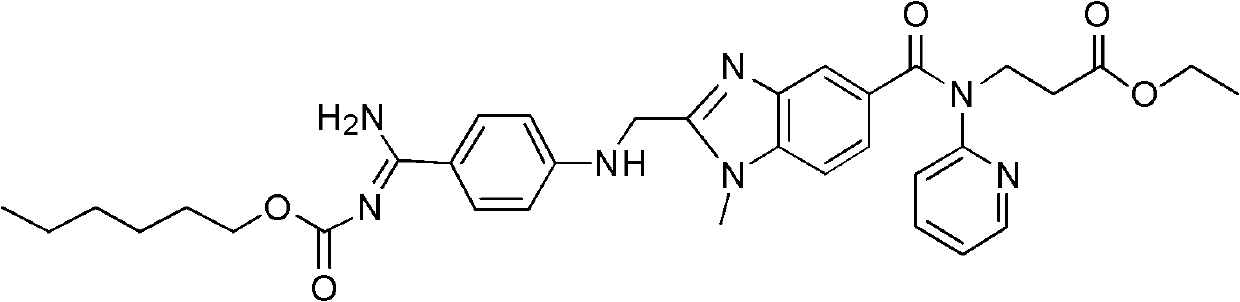
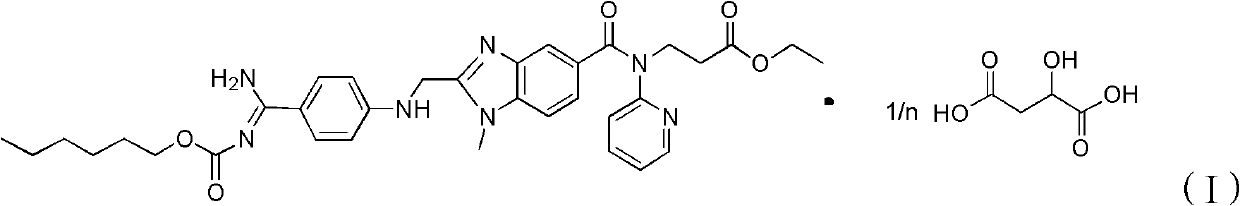
Dabigatran etexilate 2-ketoglutarate as well as preparation method and application thereof
InactiveCN102633777AImprove stabilityGood water solubilityOrganic active ingredientsOrganic chemistryPharmaceutical drugCombinatorial chemistry
The invention provides dabigatran etexilate 2-ketoglutarate shown in a general formula (I), hydrate and / or a solvate thereof. In the formula (I), n is 1, 2 or 3. The invention also provides a preparation method of dabigatran etexilate 2-ketoglutarate as well as hydrate and / or the solvate thereof and application in preparation of medicaments for treating or preventing cardiovascular diseases.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
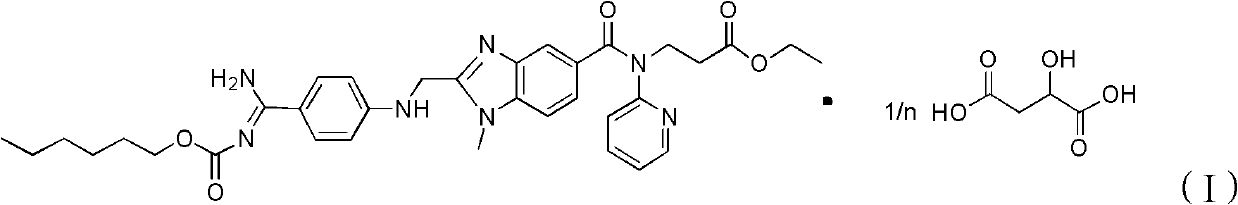
Dabigatran etexilate malate, and preparation method and application thereof
InactiveCN103304539AIncrease dissociationGood water solubilityOrganic active ingredientsCarboxylic acid salt preparationMedicineSolvent
The invention provides a dabigatran etexilate malate having a general formula shown in the specification, and a hydrate and / or solvate thereof. In the formula, n is 1, 2 or 3. The invention also provides a preparation method of the dabigatran etexilate malate, the hydrate and / or solvate thereof, and an application in the preparation of medicines for treating or preventing cardiovascular diseases.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
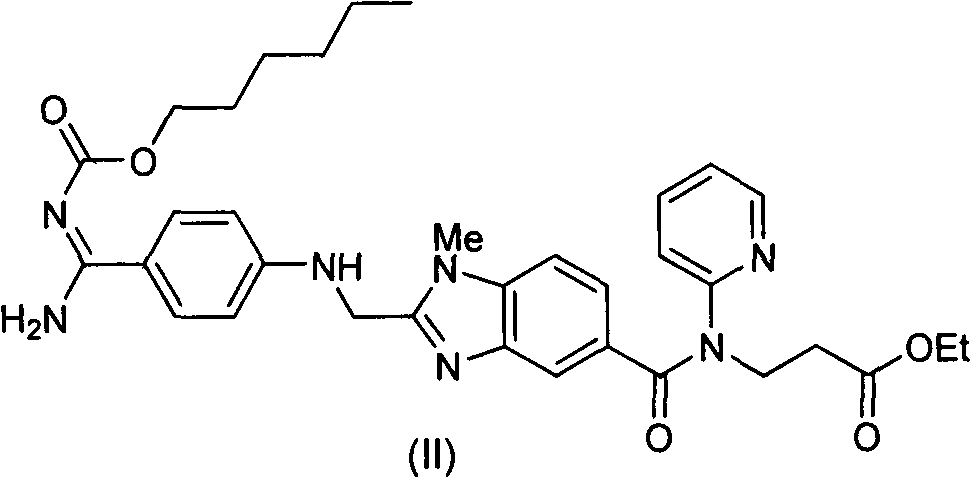
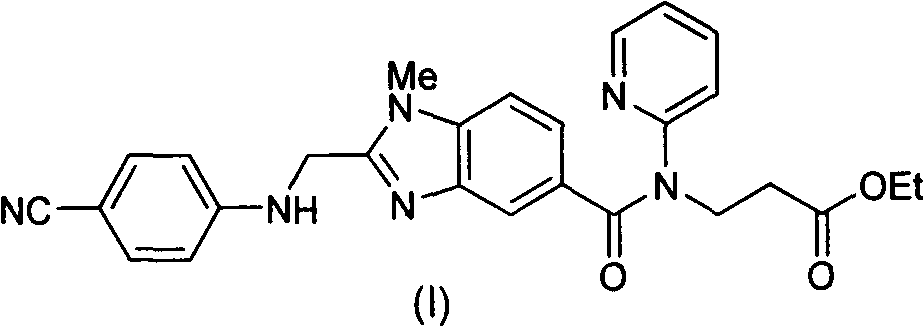
Method for preparing and purifying dabigatran etexilate intermediate
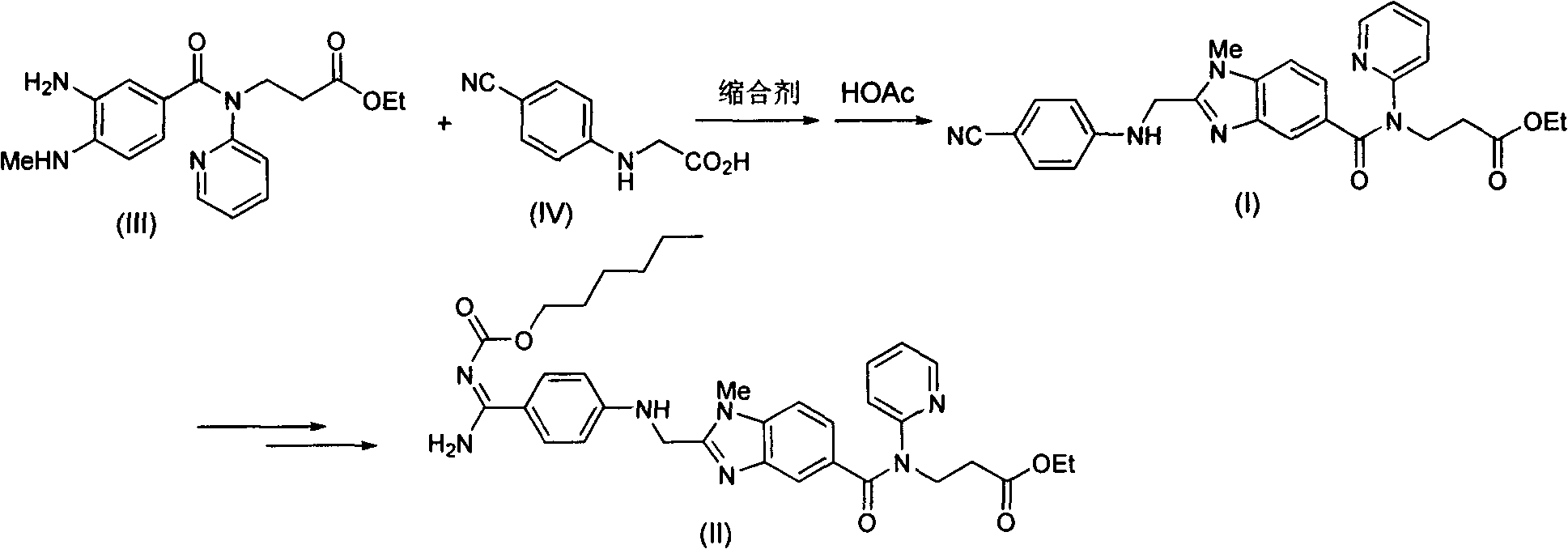
The invention discloses a method for preparing and purifying a dabigatran etexilate key intermediate 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazolyl-5-[N-(2-pyridyl)formamido]}-ethyl propionate (I). The preparation process comprises the following steps: reacting 2-( 4-cyanoanilino)acetic acid (IV) and 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamido-]-ethyl acrylate (III) in the presence of a condensing agent to form a condensate; and carrying out cyclization reaction under the catalytic action of acetic acid to prepare the 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazolyl-5-[N-(2-pyridyl)formamido]}-ethyl propionate (I). The purification process comprises the following steps: reacting the compound (I) and succinic acid to form 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazolyl-5-[N-(2-pyridyl)formamido]}-ethyl propionate succinate (V), and finally, adding alkali for dissociation to obtain the high-purity compound (I).
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Oral pharmaceutical compositions of dabigatran etexilate
Compositions comprising a mixture of at least two types of particles wherein a) the first type of particles comprise dabigatran etexilate in the form of the free base or in the form of pharmaceutically acceptable salts, polymorphs, solvates or hydrates thereof; and b) the second type of particles comprise at least one pharmaceutically acceptable organic acid, use of said compositions in the reduction of the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation and / or in the prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery and processes for the preparation of said compositions.
Owner:BRECKENRIDGE PHARM INC
Anticoagulant antidotes
The present invention relates to antibody molecules against anticoagulants, in particular dabigatran, and their use as antidotes of such anticoagulants.
Owner:BOEHRINGER INGELHEIM INT GMBH
Oral pharmaceutical composition comprising dabigatran etexilate
The present invention relates to an oral pharmaceutical composition comprising dabigatran etexilate or a pharmaceutically acceptable salt thereof, methods for preparing it and dosage forms for oral administration comprising said composition. The pharmaceutical composition is particularly useful as a medicament, especially as anticoagulant.
Owner:HEXAL AG
Dispersion preparation containing dabigatran etexilate
InactiveCN103638000ADisintegrates quicklyShort disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityPrill
The invention relates to a dispersion preparation containing dabigatran etexilate. The dispersion preparation mainly comprises a dispersible tablet containing an active substance dabigatran etexilate, and a dispersed particle capsule. The preparation has the characteristics that the preparation can be disintegrated into fine particles in water within three minutes and a uniform suspension is formed. The particles can be evenly distributed and can pass through a 2# sieve (24 meshes). The dissolution rate and the absorption rate of the medicines can be significantly increased after the medicines with poor water solubility are prepared into the dispersion preparation, and the bioavailability is also improved. Meanwhile, the dispersion preparation has the advantages of being convenient to swallow, simple in production technology, high in reproducibility, high in medicine loading rate, easy to divide the dose and the like, and does not change the pH of gastric juice of a sufferer.
Owner:CHINA PHARM UNIV
Synthesis method of dabigatran etexilate
InactiveCN104650037AEasy to routeRaw materials are easy to getOrganic chemistrySynthesis methodsChloroformate
The invention relates to the technical field of medicine synthesis and purification, in particular to a synthesis method for preparing dabigatran etexilate. The method comprises the following steps: with N-(4-cyanophenyl) glycine and 3-[(3-amino-4-methylamino benzoyl) (pyridine-2-yl) amino] ethyl propionate as raw materials, carrying out dehydration-condensation reaction, sequentially introducing hydrogen chloride and an ammonia gas to the reactants in an ethanol solution; adding hexyl chloroformate, and carrying out condensation reaction; and carrying out recrystallization to obtain the dabigatran etexilate of which the purity is 99%. The method is simple, safe, easy to operate, short in reaction cycle, and relatively high in yield and purity; impurities are easy to remove; column chromatography isolation is not needed in purification; and the method is suitable for industrialized production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
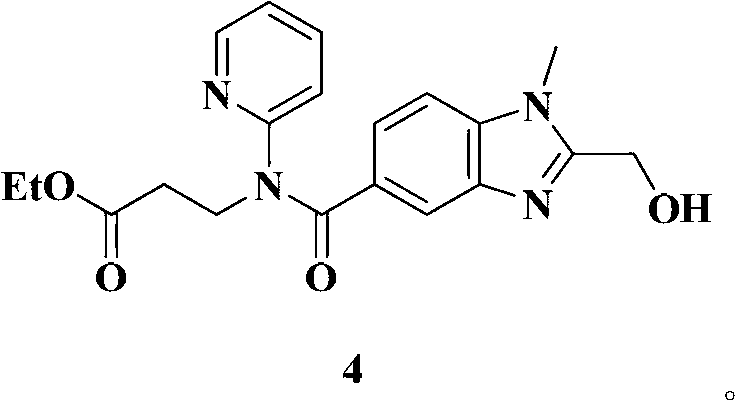
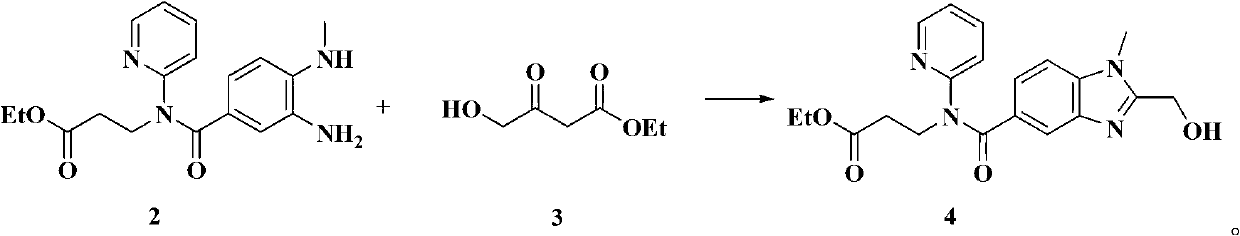
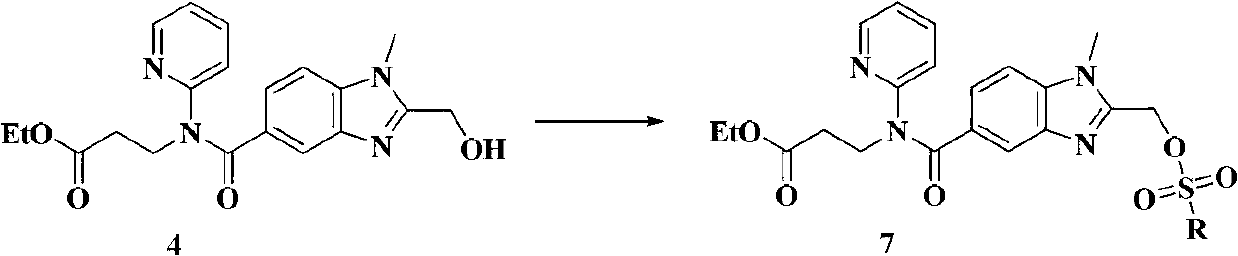
Dabigatran etexilate novel intermediate, and preparation method and application thereof
The invention belongs to the technical field of Dabigatran etexilate preparation method. The invention provides a novel compound represented by the formula 4. A preparation method of the compound is simple, and reaction conditions are mild. With the compound represented by the formula 4, a target compound Dabigatran etexilate can be obtained with two-step reactions and with high yield. Compared with prior arts, the prices of reagents adopted by the preparation method are cheap, reaction times in the steps are short, the yield is high, the conditions are mild, and intermediate purification is convenient. The method provided by the invention is a good method for preparing Dabigatran etexilate with high yield and low cost.
Owner:SHANXI WEIQIDA PHARMA IND
Anticoagulant antidotes comprising antibodies that bind dabigatran and/or related compounds and methods of use thereof
ActiveUS8821871B2Improved physicochemical propertyImprove solubilityOrganic active ingredientsPeptide/protein ingredientsAntidoteAnticoagulant
The present invention relates to antibody molecules against anticoagulants, in particular dabigatran, and their use as antidotes of such anticoagulants.
Owner:BOEHRINGER INGELHEIM INT GMBH
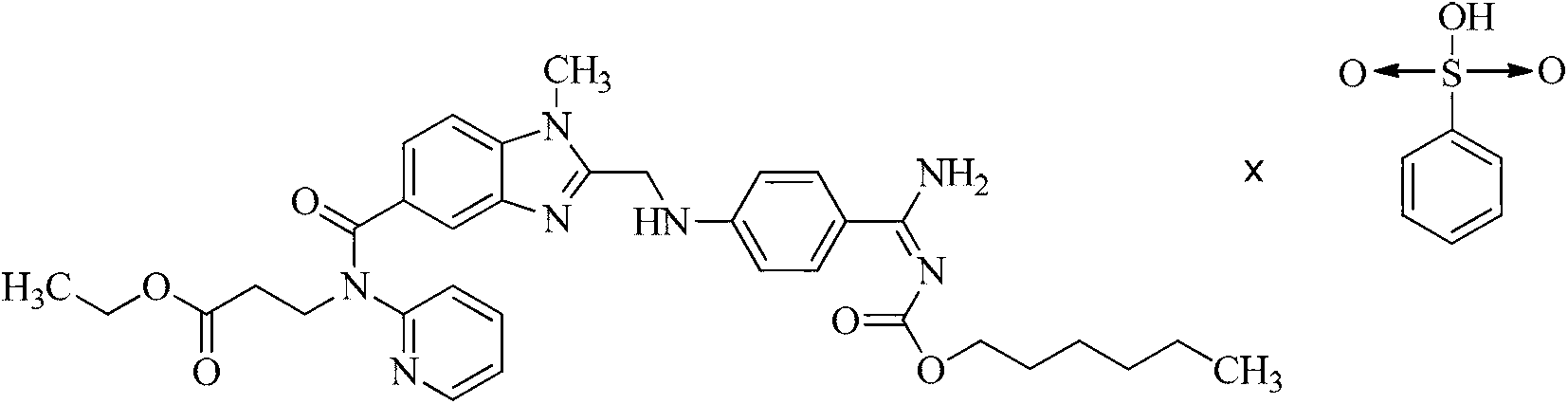
Dabigatran etexilate benzene sulfonate as well as preparation method and application thereof
ActiveCN103288800AChange physical and chemical propertiesImprove efficacyOrganic active ingredientsSulfonic acids salts preparationChemical propertyDabigatran
The invention discloses Dabigatran etexilate benzene sulfonate as well as a preparation method and application thereof. The Dabigatran etexilate benzene sulfonate has a structure shown in a formula I in the specification. According to the Dabigatran etexilate benzene sulfonate, the active substance Dabigatran etexilate is salified to change the physical and chemical properties of the active substance, and then effect of a medicament is significantly improved under the condition without pharmacokinetic differences, so that the Dabigatran etexilate benzene sulfonate has a wide prospect in the aspect of preparing an anticoagulant medicament.
Owner:HUAREN PHARMACEUTICAL CO LTD
Dabigatran etexilate liposome
ActiveCN103536535ASmall side effectsGood effectOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolHalf-life
The invention provides dabigatran etexilate liposome. The dabigatran etexilate liposome is characterized by consisting of at least one phospholipid and a lipophilic compound. The dabigatran etexilate liposome preferably comprises the following components in parts by weight: 1 part of dabigatran etexilate, 10 to 50 parts of lecithin and 1 to 10 parts of cholesterol. The invention also provides a dabigatran etexilate liposome solid preparation. The dabigatran etexilate liposome solid preparation is prepared from the following components in parts by weight: 1 part of dabigatran etexilate liposome, 1 to 3 parts of a filling agent, 0 to 0.2 part of a disintegrating agent, 0.02 to 0.1 part of an adhesive and 0 to 0.08 part of a lubricating agent. The solid preparation can also comprise 0 to 10 parts of a corrigent and 0 to 0.15 part of a flavoring agent. According to the technical scheme of the invention and the advantages of the liposome, the medicine adsorption and utilization ratio of dabigatran etexilate is increased, the half-life period of the medicine is prolonged, and the administration dosage or the administration frequency can be effectively reduced.
Owner:江苏阿尔法集团盛基药业(宿迁)有限公司
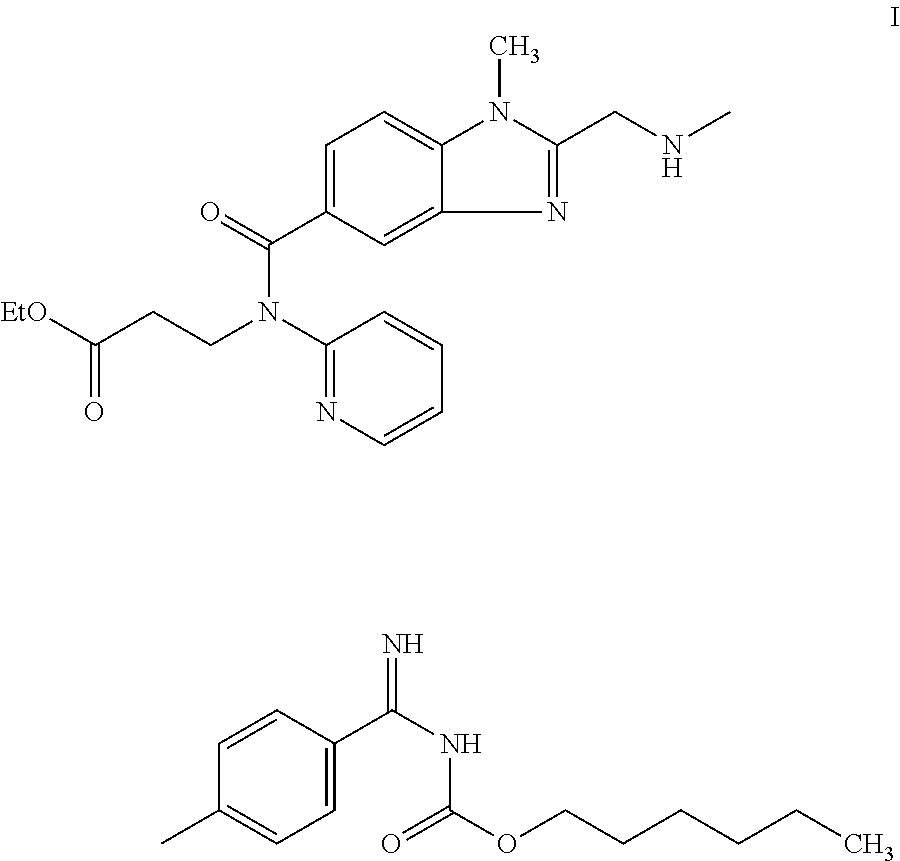
Dabigatran etexilate derivative and preparation method and application thereof
ActiveCN102766134AImprove oral bioavailabilityStrong anticoagulant inhibitory effectOrganic active ingredientsOrganic chemistryRacemic mixtureDabigatran
The invention provides a dabigatran etexilate derivative shown as a formula I, or pharmaceutically acceptable salt, a solvate, a polymorph, an antipode or a racemic mixture thereof. In the formula I, R1 is hydrogen or C1 to C5 alkyl, R2 is shown in the specifications, R3 and R4 are independently hydrogen or C1 to C5 alkyl, n is 0 or 1, and R5 is C1 to C8 alkyl or optionally substituted C1 to C8 alkyl. The compound has the activity of a thrombin inhibitor. The invention also provides a preparation method for the compound, a compound-containing medical composition, and application of the compound and the medicinal composition to preparation of thrombin inhibitor medicines and treatment of related diseases.
Owner:BEIJING PRELUDE PHARM SCI & TECH
Improved method for preparing Dabigatran etexilate
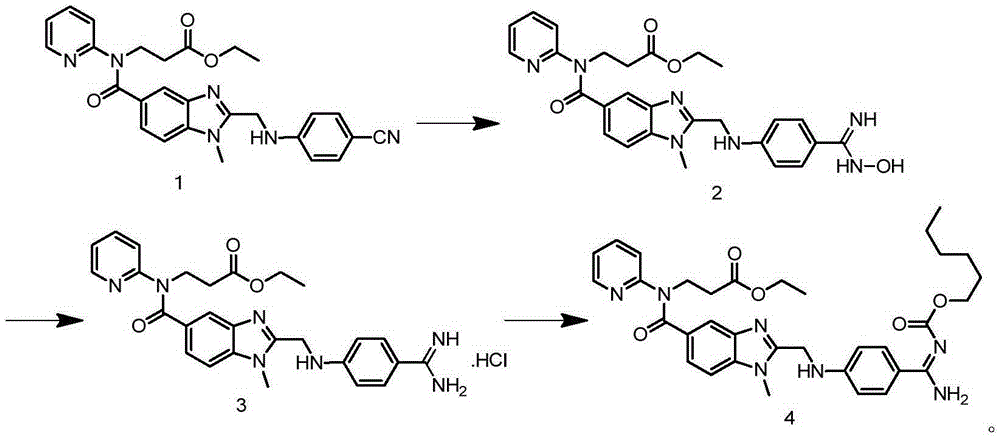
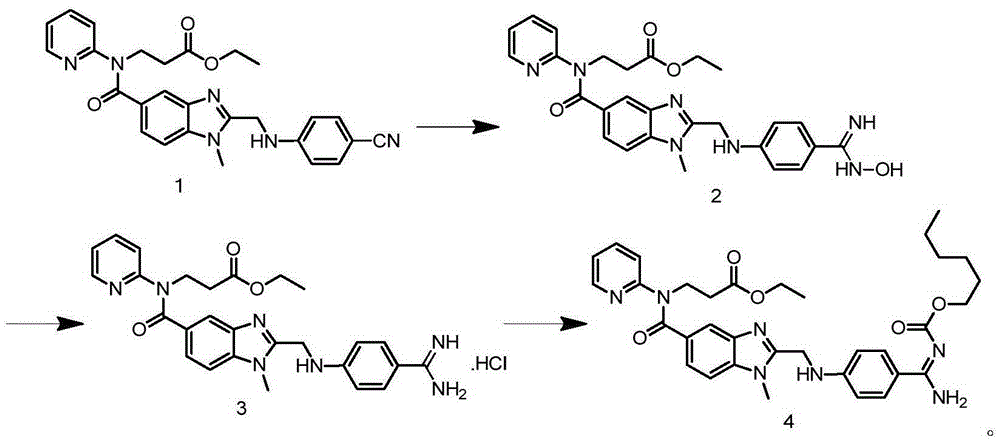
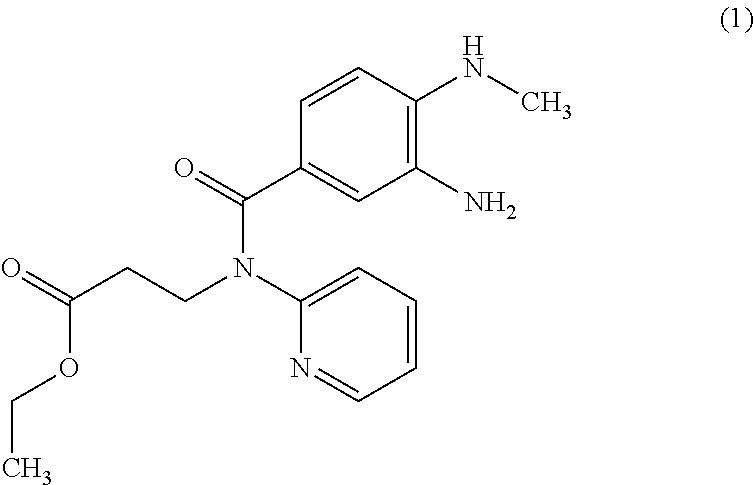
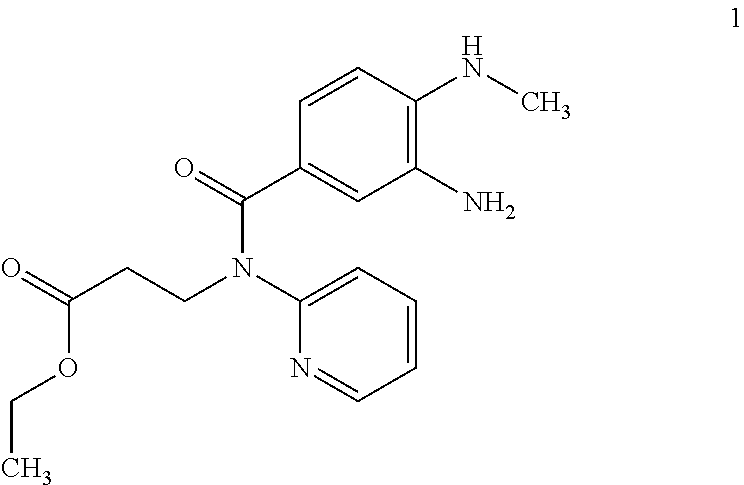
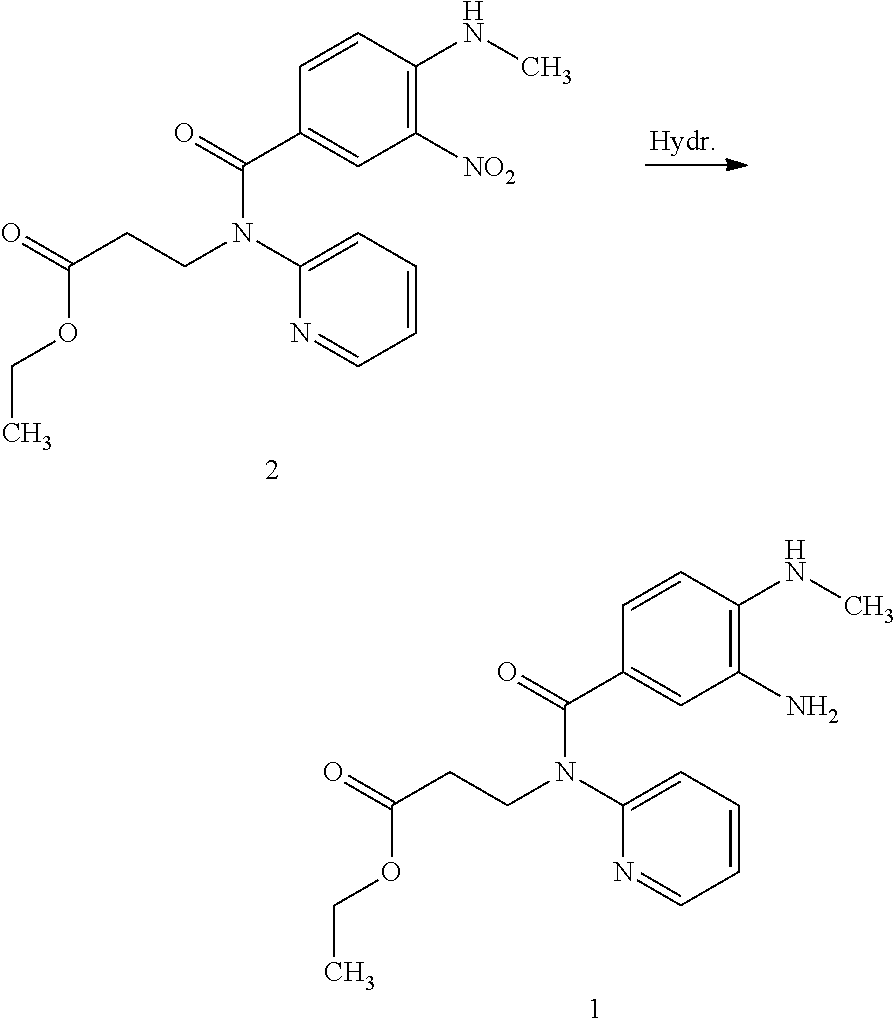
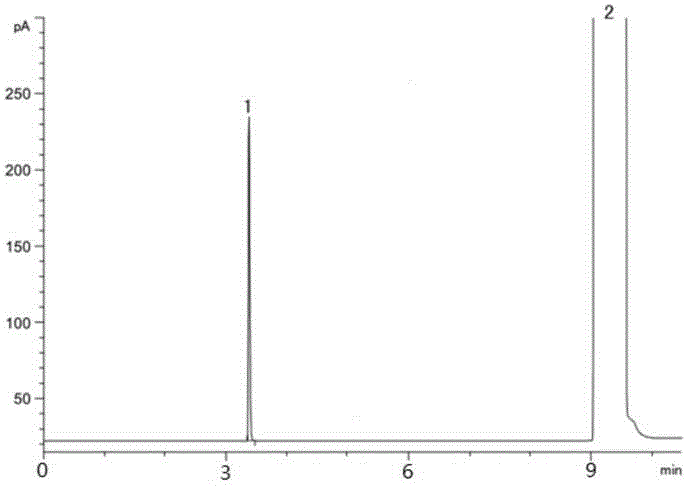
The invention relates to a method for preparing Dabigatran etexilate. The method comprises the specific steps of enabling a compound represented by a formula 1 shown in the description, i.e., 3-[[[2-[[(4-cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl] pyrid-2-ylamino]ethyl propionate to subject to addition reaction with hydroxylamine hydrochloride in the presence of a catalyst in an alcoholic solution, so as to obtain a compound represented by a formula 2 shown in the description, reducing the compound represented by the formula 2 so as to obtain a compound represented by a formula 3 shown in the description, and enabling the compound represented by the formula 3 to subject to amidation reaction with n-hexyl chloroformate in the presence of a catalyst, thereby obtaining a compound represented by a formula 4 shown in the description, i.e., Dabigatran etexilate. Compared with the prior art, the preparation process disclosed by the invention has the advantages that the generation of a large volume of waste acid in the prior art is avoided, the reaction conditions are mild, the control is easy, the yield is high, and the product quality is good, thereby being applicable to industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
Anticoagulant antidotes
ActiveUS20120276123A1Improved physicochemical propertyImprove solubilityOrganic active ingredientsAntinoxious agentsAnticoagulantDabigatran
The present invention relates to antibody molecules against anticoagulants, in particular dabigatran, and their use as antidotes of such anticoagulants.
Owner:BOEHRINGER INGELHEIM INT GMBH
Dabigatran etexilate analogue with fluorine-containing group modified pyridine ring as center and synthesis method of analogue
InactiveCN103242296AEffective combinationReduced bioavailabilityOrganic chemistryBlood disorderCombinatorial chemistryAminopyridines
The invention discloses a dabigatran etexilate analogue with fluorine-containing group modified pyridine ring as a center and a synthesis method of the analogue. According to the synthesis method, with a fluorine-containing group aminopyridine compound as a starting material, a series of reactions are carried out to prepare he fluorine-containing group modified dabigatran etexilate analogue. The synthesis method of the analogue has the advantages that the operation is simple, used reagents are low in cost and easily available, synthesis cost is relatively low, rick in the synthetic process is low, yield of each synthesis step is high, and synthesis time is short.
Owner:SHANGHAI INST OF TECH
Emergency interventions of active charcoal with dabigatran etexilate overdosing
InactiveUS20110206656A1BiocideOrganic active ingredientsPharmaceutical medicineIntensive care medicine
The invention relates to a method for treating an overdosing with active substance dabigatran etexilate of formula Ioptionally in the form of the pharmaceutically acceptable salts thereof, comprising the administration of an effective amount of charcoal.
Owner:BOEHRINGER INGELHEIM INT GMBH
Dabigatran derivatives as well as preparation method and application thereof
ActiveCN104592204AImprove bioavailabilityGood effectOrganic active ingredientsOrganic chemistryMedicinal chemistryDabigatran
The invention belongs to the field of medicines, and specifically relates to dabigatran derivatives or the pharmaceutical salts thereof, a preparation method for the derivatives, a medicine composition containing the dabigatran derivatives, and an application of the derivatives and the medicine composition in preparation for anticoagulant medicines and treatment for related diseases.
Owner:CHINA RESOURCES SAIKE PHARMA
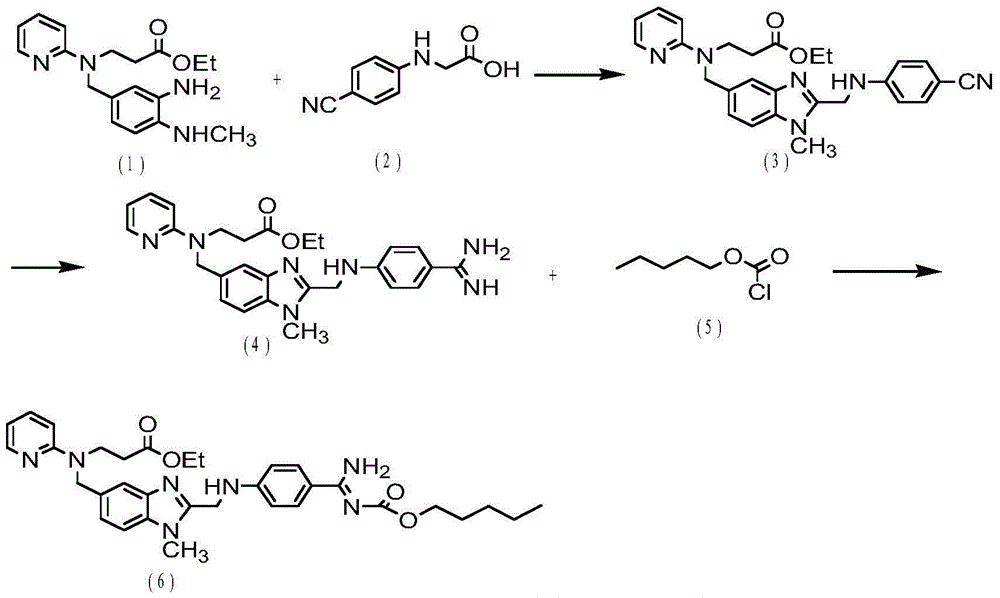
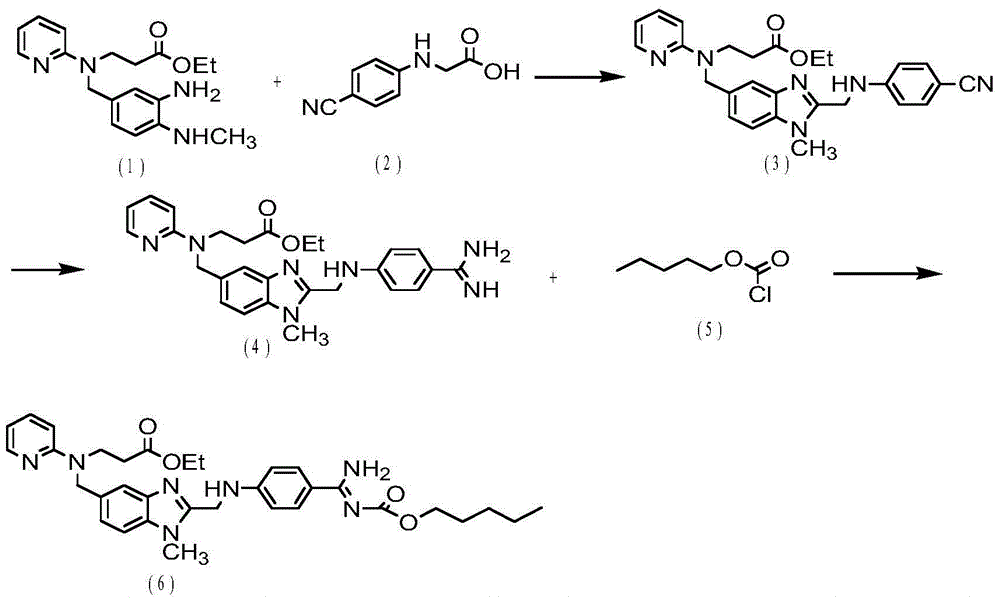
Dabigatran etexilate preparation method
ActiveCN104031031ASuitable for large-scale industrial productionRaw materials are cheap and easy to getOrganic chemistryStereochemistryRaw material
The invention discloses a dabigatran etexilate preparation method. The method comprises the following steps: preparing an intermediate 1, preparing an intermediate 2, preparing an intermediate 3, preparing an intermediate 4, preparing an intermediate 5, preparing an intermediate 6, and preparing dabigatran etexilate. The preparation method has the advantages of cheap and easily available raw materials, easy operation, easy control, high reaction yield, high product purity, and suitableness for the large-scale industrial production of dabigatran etexilate.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Process for the manufacture of an intermediate in the synthesis of dabigatran
The invention relates to a process for the synthesis of the diamine of formula (1) an important intermediate in the synthesis of dabiagtran etexilate.
Owner:BOEHRINGER INGELHEIM INT GMBH
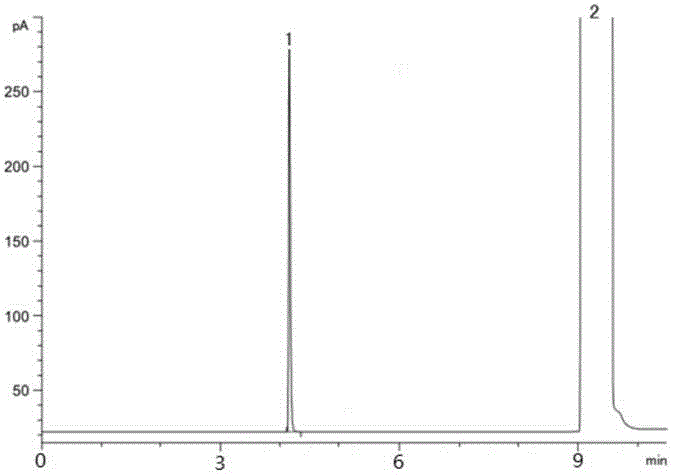
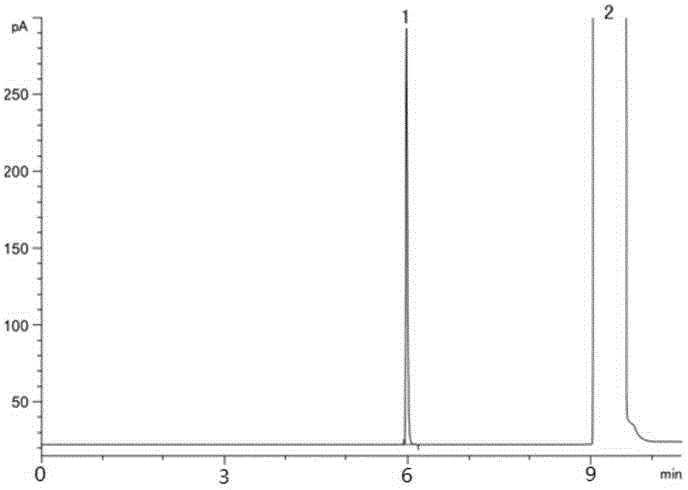
Method for detecting organic-solvent residues in dabigatran etexilate
The invention relates to a method for detecting organic-solvent residues in dabigatran etexilate, and particularly relates to a method for simultaneously detecting residues of alcohol, acetone, ethyl acetate and tetrahydrofuran in dabigatran etexilate by gas chromatography. An external standard method is adopted for simultaneously detecting the amount of the residues of four types of organic solvents in the dabigatran etexilate, and the chromatographic conditions are as follows: a chromatographic column is in the type of Agilent DB-1301 (30m*0.32mm*0.25microns), the column temperature is increased by programming, the initial temperature is 40 DEG C, the maintaining time is 6 minutes, then the temperature is increased to be 180 DEG C at a speed of 50 DEG C / min and is maintained for 10 minutes, the temperature of a sample inlet is 200 DEG C, the detection temperature is 250 DEG C, carrier gas is N2, the flow is 2ml / min, a headspace sampling method is adopted, the balance time is 30 minutes and the balance temperature is 90 DEG C. The method is high in sensitivity, good in repeatability and high in accuracy, and is suitable for detecting the organic-solvent residues in the dabigatran etexilate.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
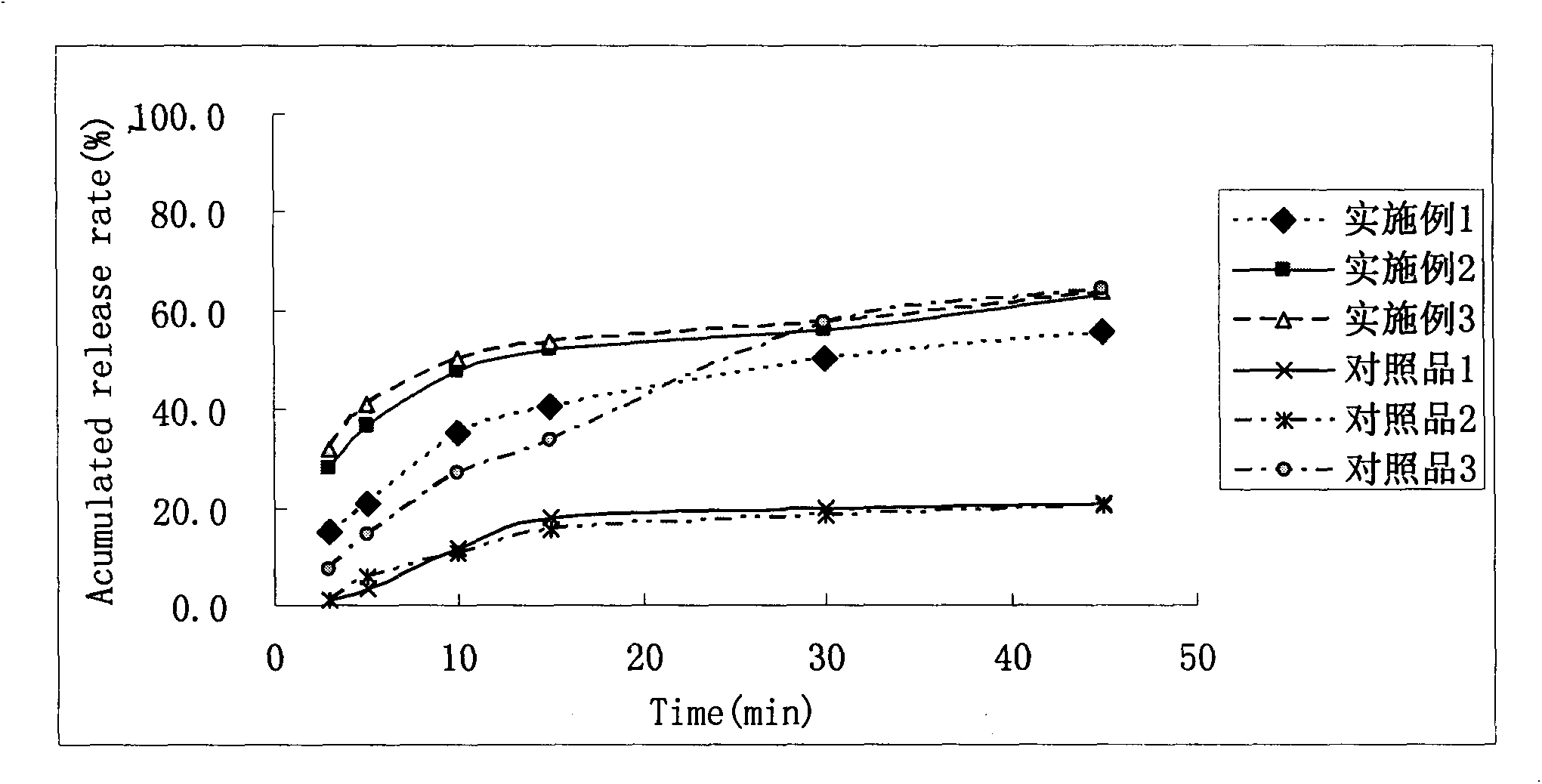
Oral double-pellet pharmaceutical composition of dabigatran or its salt
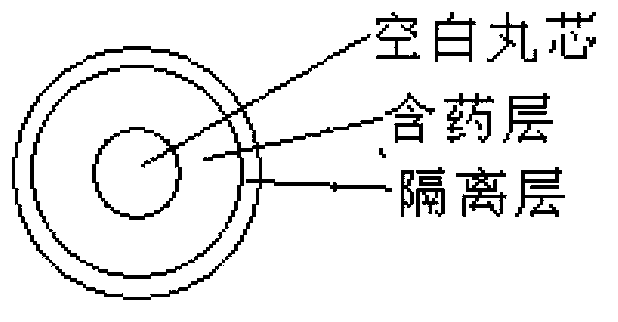
ActiveCN104274444ATroubleshooting Dissolution Rates That Are Lower Than ExpectedHigh degree of dissolutionOrganic active ingredientsPill deliverySolubilityAdhesive
This invention relates to an oral double-pellet pharmaceutical composition of dabigatran or its salt. Specifically, the double-pellet pharmaceutical composition comprises: a) a spherical or sphere-like medicated pellet composed of an active substance, a pharmaceutically common pore-foaming agent, and a disintegrating agent and / or an adhesive, with the medicated pellet wrapped by an isolation layer formed by a water soluble polymer; and b) an organic acid pellet containing the adhesive and / or the pore-forming agent and / or the disintegrating agent, with the water solubility of the organic acid at 20DEG C being greater than 1g / 250ml. The two pellets are mixed evenly according to proportion and then loaded into a capsule shell, or a pharmaceutically common diluent and / or a pore-forming agent and / or a disintegrating agent and / or a lubricant are / is added to be pressed into tablets. The pharmaceutical composition has the advantages of simple preparation process, good reproducibility, good in vitro dissolution and higher in vivo bioavailability.
Owner:JIANGSU HANSOH PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com