Dabigatran etexilate derivative and preparation method and application thereof
A technology of dabigatran etexilate and its derivatives, which is applied in the field of medicine and can solve the problems of low oral bioavailability of dabigatran etexilate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
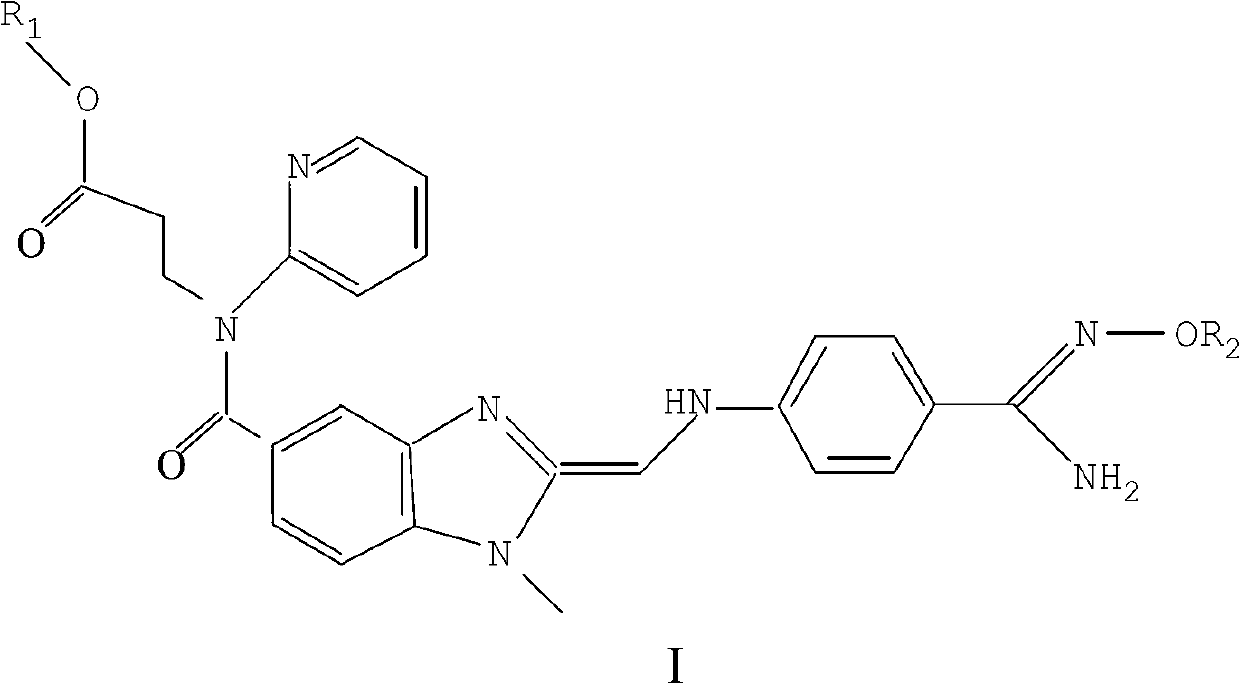
[0057] Embodiment 1: the preparation of compound I2 of the present invention
[0058]
[0059] step 1):
[0060]
[0061] To a stirred solution of Formula III (2.0 g, 4.15 mmol) in EtOH (50 mL) was added hydroxylamine hydrochloride (1.72 g, 24.9 mmol) and DIPEA (4.36 mL, 24.9 mmol). The mixture was stirred at 90°C for 16 hours. The solution was cooled to room temperature, then the solvent was removed under reduced pressure. The residue was partitioned between EA (50 mL) and water (20 mL), and the organic layer was separated. The aqueous phase was further extracted with EA (2 x 30 mL) and the combined organic extracts. Dry over NaSO4, filter and concentrate. The crude product (550 mg, 25.7%) was purified on a silica gel column MW1207.
[0062] 1H NMR(DMSO-d6,400MHz)δ9.24(s,1H),8.38-8.39(m,1H),7.37-7.56(m,5H),7.09-7.15(m,2H),6.88(d,J =8.0Hz,1H),6.69(d,J=8.8Hz,2H),6.44(t,J=5.2Hz,1H),5.56(br s,2H),4.51(d,J=5.6Hz,2H) ,4.22(t,J=7.2Hz,2H),3.97(q,J=7.2Hz,2H),3.76(s,3H),...
Embodiment 3
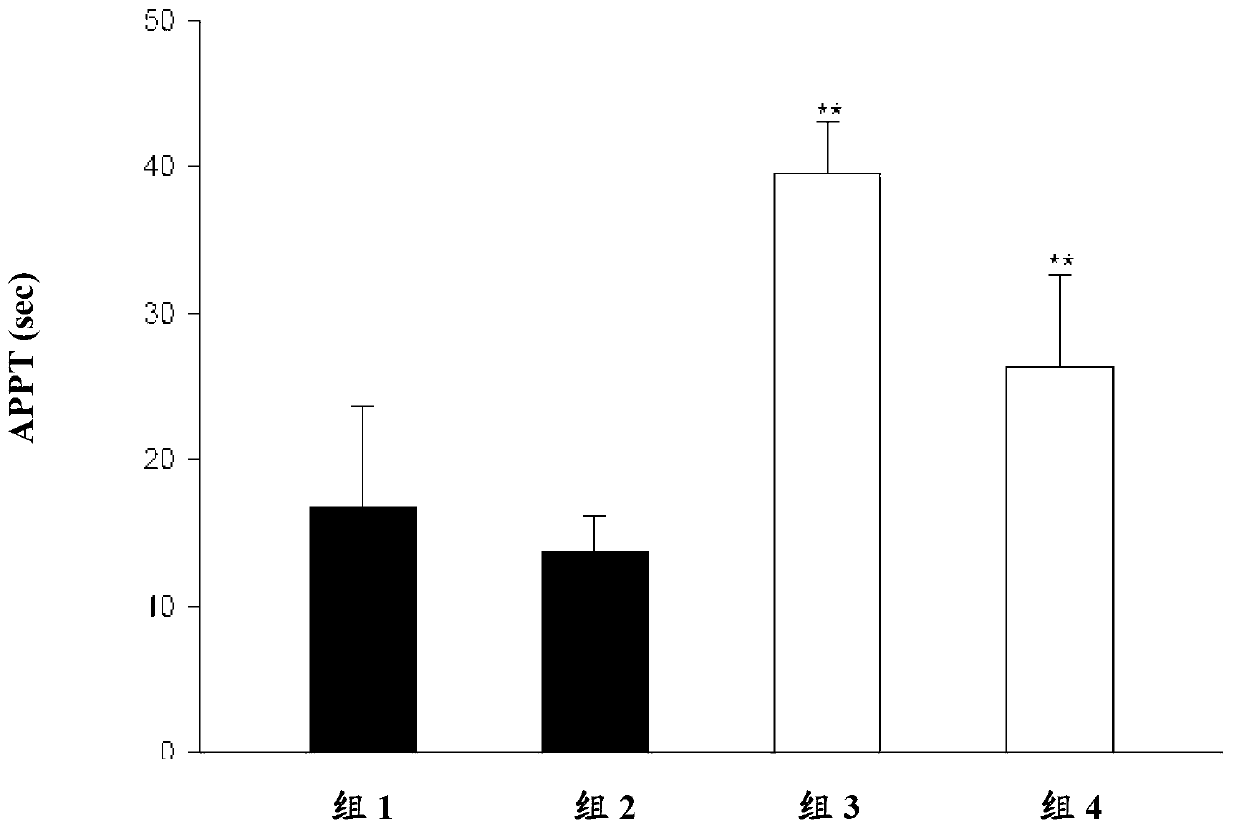
[0078] Embodiment 3: in vitro stability test
[0079] In this example, the in vitro hepatic microsomal stability of the compound of formula I1 and compound of formula I2 of the present invention was tested, wherein the formation of dabigatran was detected and compared with the known compound of formula I5.
[0080] Test compound: compound of formula I1, compound of formula I2 and compound of formula I5;
[0081] Control compound: verapamil.
[0082] Microsomes: Human liver microsomes and rat liver microsomes were purchased from CellzDirect (Invitrogen); store at –80°C until use.
[0083] method:
[0084] 1) Prepare the mother solution as shown in Table 1, and then add the test compound or control compound, so that the final concentration of these compounds in the reaction system is 2 μM. Then preheat the mixed solution at 37 °C for 2 min.
[0085] Table 1. Preparation of mother liquors
[0086]
[0087] 2) NADPH was added to the mixed solution to make the final conce...
Embodiment 4
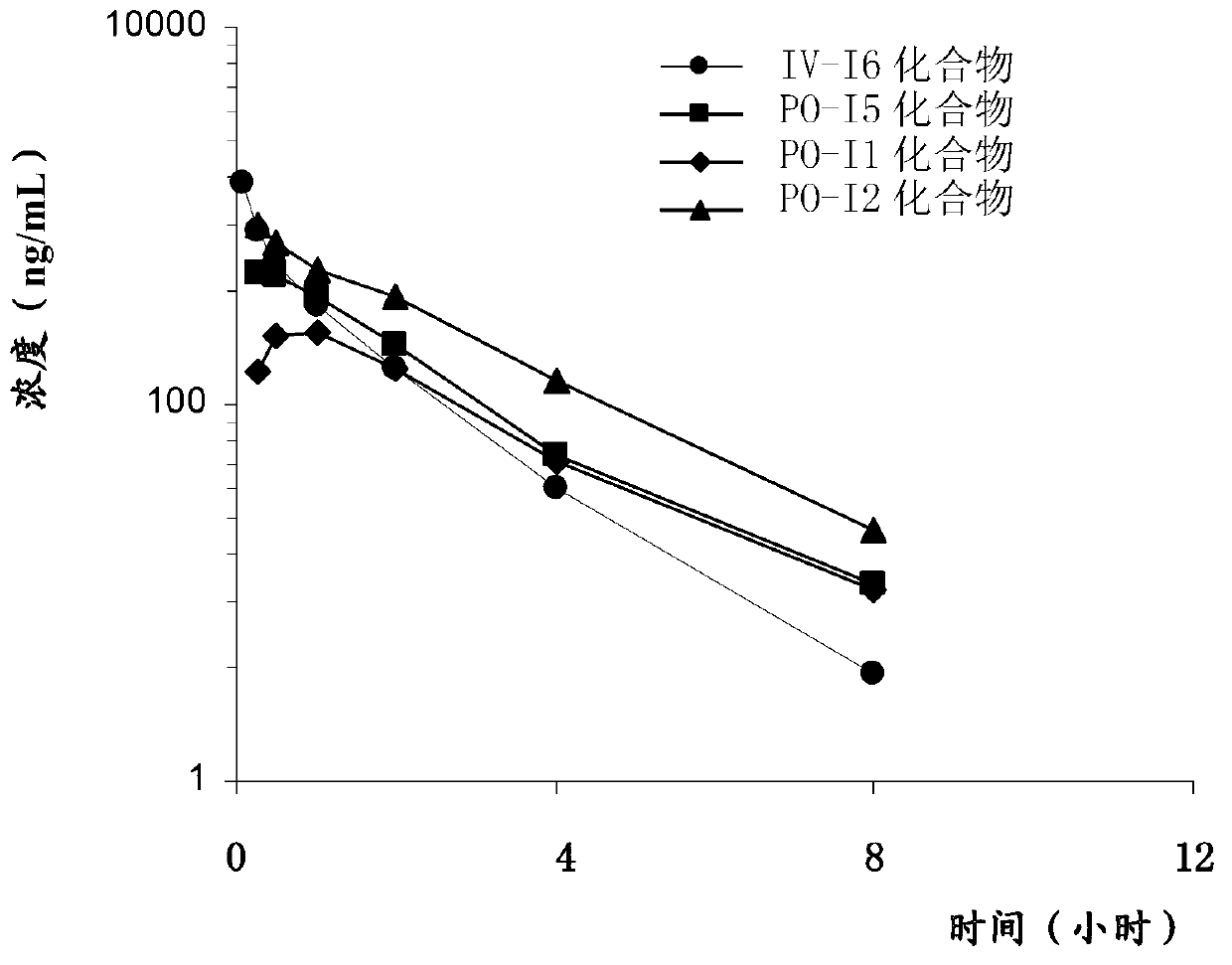
[0110] Embodiment 4: Pharmacokinetic test in vivo
[0111] In this embodiment, the in vivo pharmacokinetics of compounds I1, I2 and I6 of the present invention and the known dabigatran etexilate (formula I5) were detected.
[0112] method:
[0113] Compounds I1, I2, I5 and I6 were dissolved in the blank solution (30% PEG-400 and saline) at a concentration of 1 g / L, respectively.
[0114] The experimental animals were male SD rats, aged 6 to 8 weeks, weighing 190-215 grams, purchased from Beijing Weili Tonghua Experimental Animal Technology Co., Ltd. SD rats were randomly divided into 4 groups based on body weight, with 3 animals in each group. See Table 4 for the dosage and route of each group of rats.
[0115] Table 4. Grouping and administration of pharmacokinetic tests
[0116]
[0117] Before the pharmacokinetic test, SD rats were fasted for 16 hours. A single dose of compound or blank solution was then administered intravenously (1 mg / kg) or orally (10 mg / kg) as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com