Dabigatran etexilate intermediate, preparation method for same and method for preparing dabigatran etexilate
A technology of dabigatran etexilate and intermediates, applied in the field of dabigatran etexilate intermediates and its preparation, can solve the problems of low yield of intermediates, low purity of intermediates, high price of dehydrating agent, etc., and achieve product yield The effect of high yield and purity, simplified preparation process and good market prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The present invention also provides a preparation method of the above-mentioned dabigatran etexilate intermediate, comprising the following steps:
[0063] The compound of formula (1) is acylated with the compound of formula (A) to obtain the aforementioned dabigatran etexilate intermediate (that is, the compound of formula (2));
[0064]
[0065] In formula (A), X is selected from chlorine, bromine, iodine, R is selected from hydroxyl, chlorine, -O-CH 2 CH 3 ,
[0066] Preferably, the specific process of the reaction between the compound of formula (1) and the compound of formula (A) is as follows: first drop the ethyl acetate solution of the compound of formula (A) into the tetrahydrofuran solution of the compound of formula (1) to obtain a reaction solution, and then react The liquid was reacted at a temperature of 25°C-50°C for at least 10 hours, then the reaction liquid was cooled and suction filtered, and the obtained solid was the aforementioned dabigatran e...
Embodiment 1
[0098] Example 1 Preparation of N-(3-bromoacetamido-4-methylamino)benzoyl-N-(2-pyridyl)-3-aminopropionic acid ethyl ester (i.e. X is a bromine formula (2) compound)
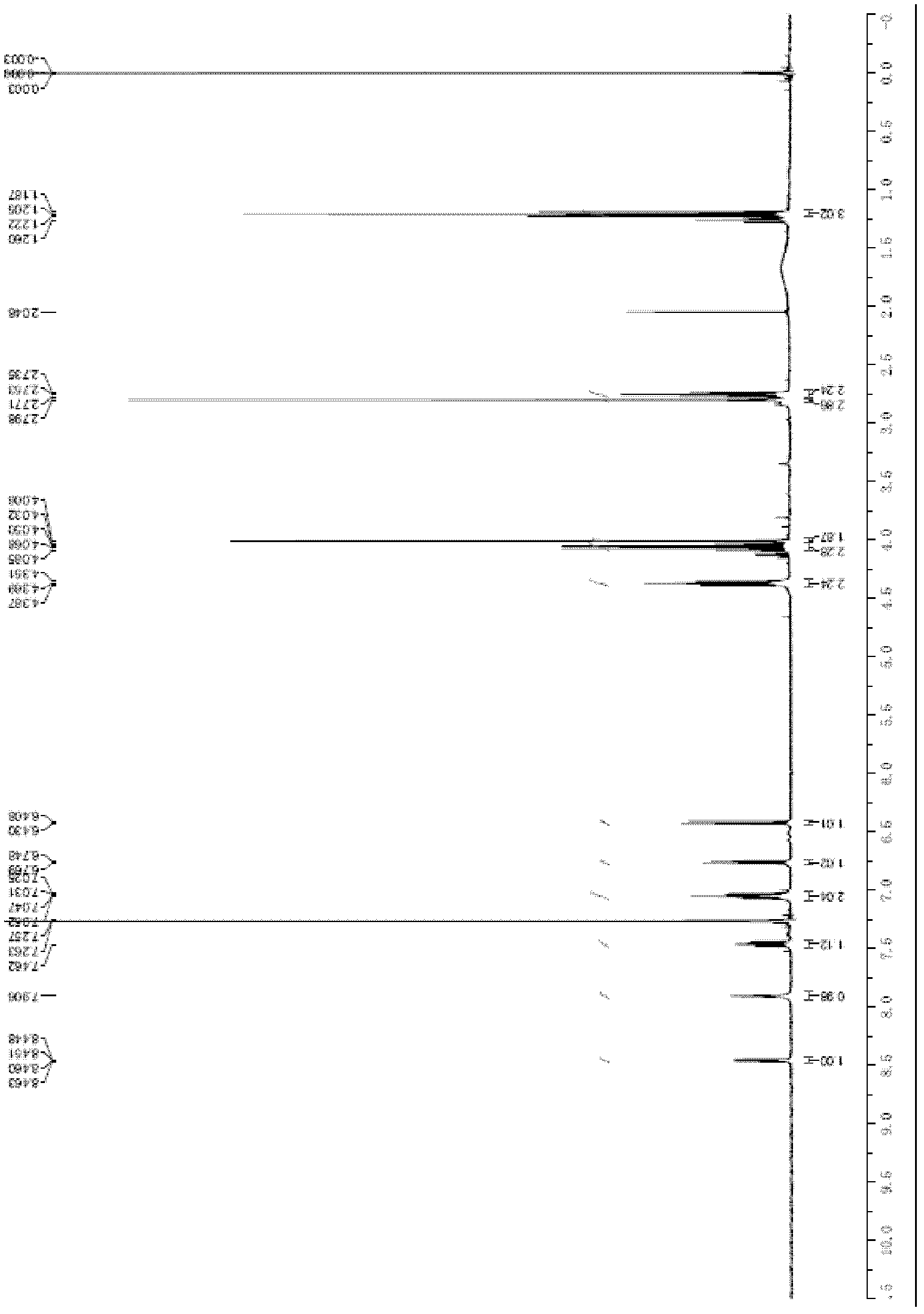
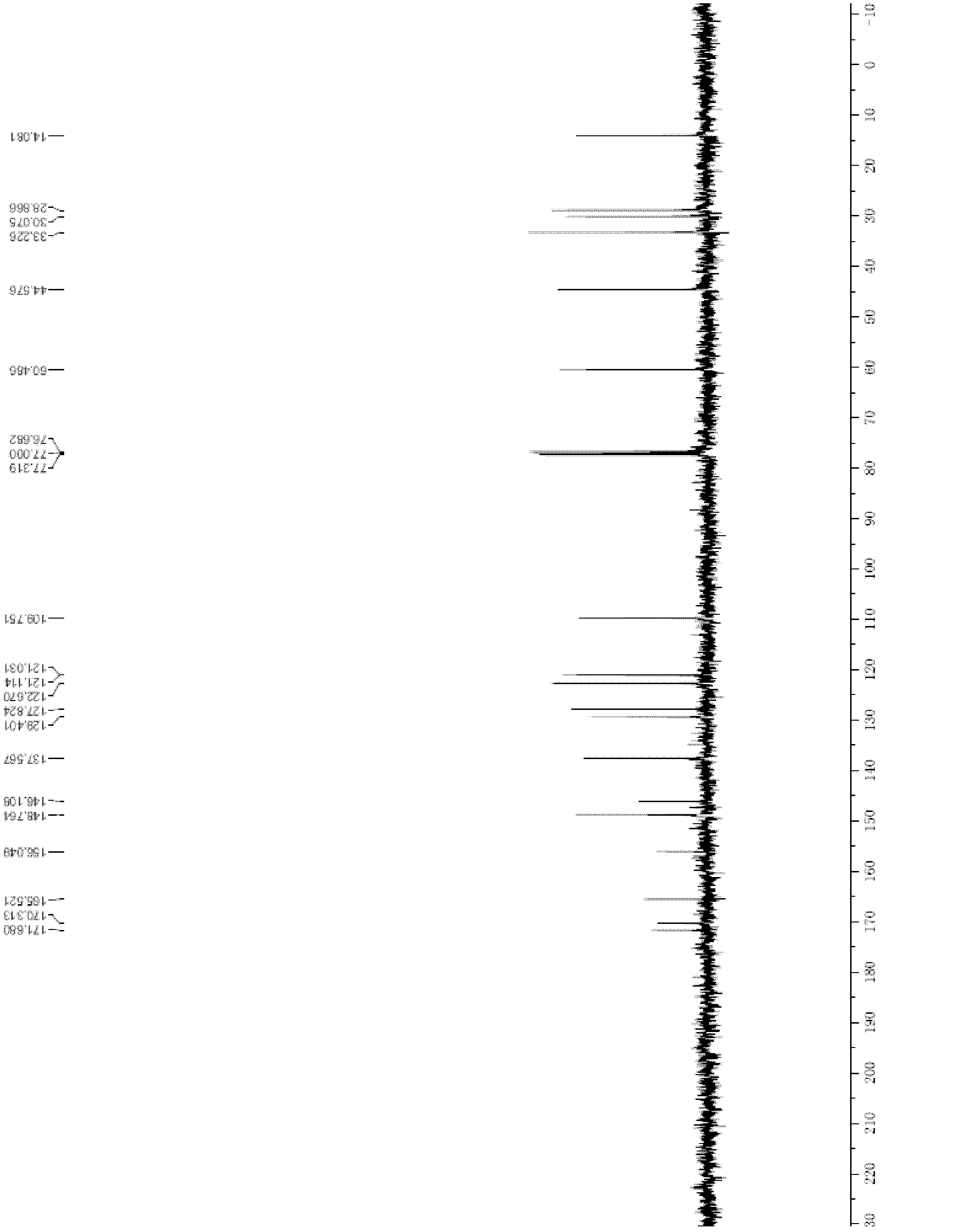
[0099] Put 130g of the compound of formula (1) and 4000ml of tetrahydrofuran into the reaction flask, stir evenly, then slowly dropwise add ethyl acetate solution (200ml) containing 109g of bromoacetic anhydride to the flask at 25°C, and then heat to 50°C to react for at least 10 Hour. Cooling and suction filtration; the filter cake was washed with tetrahydrofuran (2×100ml), and dried to obtain N-(3-bromoacetamido-4-methylamino)benzoyl-N-(2-pyridyl)-3-aminopropyl Acetate ethyl ester 166g, yield is 95.8%, HPLC purity is 98.5%, 1 H-NMR picture as figure 1 shown, 13 C-NMR picture as figure 2 shown. 1 H-NMR (CDCl 3 )δ: 1.1(t, 3H), 2.7(t, 2H), 2.7(s, 3H), 4.0(m, 2H), 4.0(s, 2H), 4.3(t, 2H), 6.4(m, 1H ), 6.7 (m, 1H), 7.0 (m, 2H), 7.2 (m, 1H), 7.9 (m, 1H), 8.4 (m, 1H). 13 C-NMR (CDCl 3 )δ: 14.0, 28.6, 30.0, 33...
Embodiment 2
[0104] Embodiment 2 prepares formula (6) compound by N-(3-bromoacetamido-4-methylamino)benzoyl-N-(2-pyridyl)-3-aminopropionic acid ethyl ester
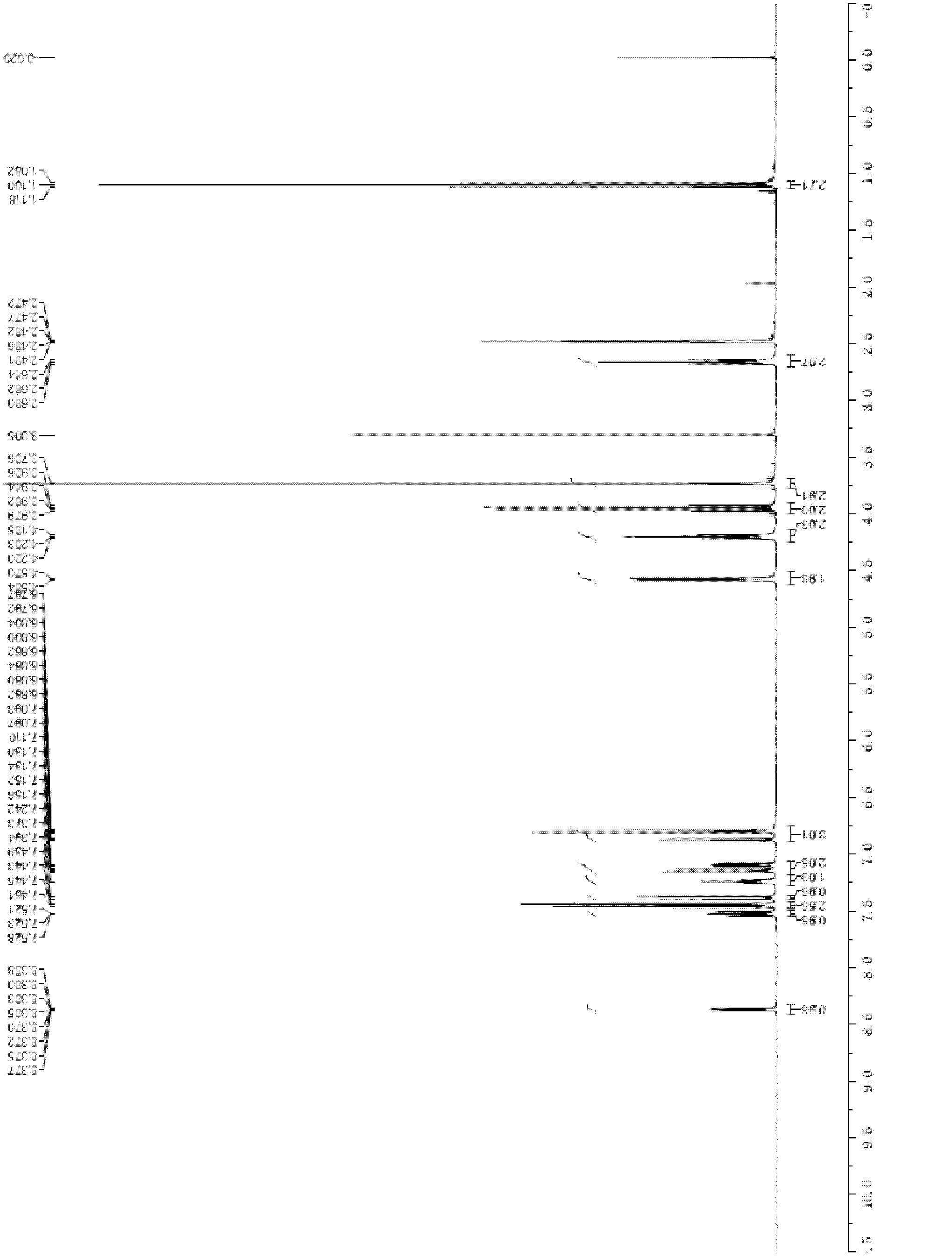
[0105] 46.2g N-(3-bromoacetamido-4-methylamino)benzoyl-N-(2-pyridyl)-3-alanine ethyl ester, 600ml dimethylformamide (DMF), 17.6g Put p-aminobenzonitrile, 5.2g potassium iodide, and 13.8g potassium carbonate into the reaction bottle, react at 40°C for 10 hours, then slowly heat to 80°C for 2 hours; then cool to room temperature, add 2500ml of water to precipitate for 0.5 hours (during this process Stir in middle), suction filtration; Filter cake is washed with water (2 * 100ml), recrystallizes from ethyl acetate (960ml) after drying, obtains 41g formula (6) compound after drying again, i.e. 3-[[[2-[[ (4-cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl)amino]propionic acid ethyl ester, yield 85% , optical purity ≥ 97%, 1 H-NMR picture as image 3 shown. 1 H-NMR (DMSO) δ: 1.1(t, 3H), 2.7(t, 2H), 3.9(s, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com