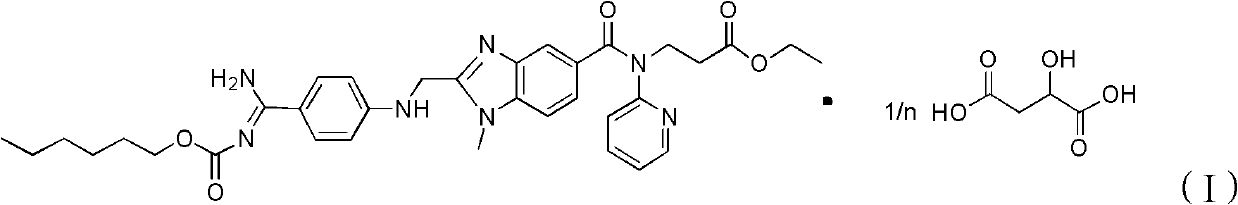
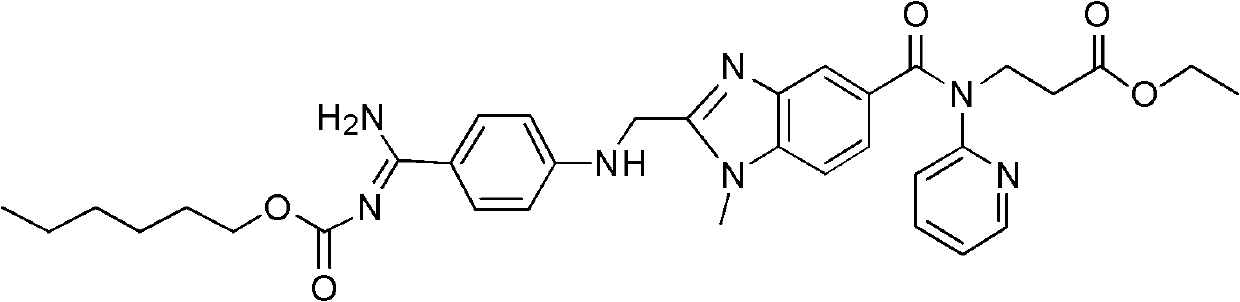
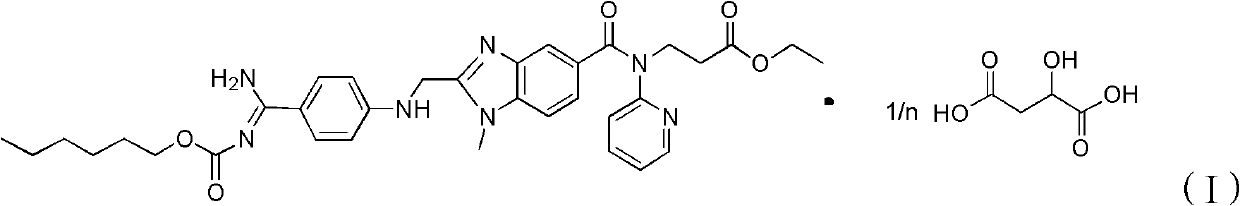
Dabigatran etexilate malate, and preparation method and application thereof
A technology of dabigatran etexilate and malate, which is applied in the field of acid addition salt of dabigatran etexilate, can solve the problems of poor stability and low bioavailability, and achieve improved water solubility, good solubility or The effects of compressibility, high stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment is used to illustrate the preparation of dabigatran etexilate malate of the present invention.
[0049] Add 1.6mmol of dabigatran etexilate and 1.6mmol of malic acid to 20ml of absolute ethanol at 20°C, mix and stir for 6 hours to form a salt, then crystallize at 20°C, filter, and wash with ethyl acetate , after drying, ether was added for recrystallization to obtain 0.310 g of ether solvate of dabigatran etexilate malate. Measured ESI-MS (electrospray ionization-mass spectrometry) (m / z): 799[M+H] + .
[0050] The ether solvate was dried to obtain 0.300 g of dabigatran etexilate malate as a white solid. According to calculation, in the ether solvate, the content of dabigatran etexilate malate was 95.4wt%. The ether solvate contained 0.5 molecule of ether per molecule.
[0051] Determination of above-mentioned dabigatran etexilate malate:
[0052] ESI-MS(m / z): 762[M+H] +
[0053] 1 H NMR (DMAO-d 6 , 400MHz) δ: 0.88 (t, J=9.0Hz, 3H, CH 3 ), (t, J=...
Embodiment 2
[0062] This embodiment is used to illustrate the preparation of dabigatran etexilate malate of the present invention.
[0063] Add 3.2mmol of dabigatran etexilate and 1.6mmol of malic acid into 20ml of water at 30°C, mix and stir for 6 hours to form a salt, then crystallize at 30°C, filter, wash with ethyl acetate, and dry , Add water to carry out recrystallization, obtain the hydrate of the dabigatran etexilate malate of 0.450g. Measured ESI-MS(m / z): 713[M+H] + .
[0064] The above-mentioned hydrate was dried to obtain 0.439 g of dabigatran etexilate malate as a white solid. According to calculation, in the above-mentioned hydrate, the content of dabigatran etexilate malate was 97.5wt%. Each molecule of the hydrate contains 1 molecule of water, that is, monohydrate.
[0065] Determination of above-mentioned dabigatran etexilate malate:
[0066] ESI-MS(m / z): 695[M+H] +
[0067] 1 H NMR (DMAO-d 6 , 400MHz) δ: 0.88 (t, J=9.0Hz, 3H, CH 3 ), (t, J=8.4Hz, 3H, CH 3 ), 1.27...
Embodiment 3
[0076] This embodiment is used to illustrate the preparation of dabigatran etexilate malate of the present invention.
[0077] Add 4.8mmol of dabigatran etexilate and 1.6mmol of malic acid to 20ml of absolute ethanol at 0°C, mix and stir for 6 hours to form a salt, then crystallize at 0°C, filter, wash with ethyl acetate, After drying, ethylene glycol dimethyl ether was added for recrystallization to obtain 0.420 g of dabigatran etexilate malate as a white solid.
[0078] Determination of above-mentioned dabigatran etexilate malate:
[0079] ESI-MS(m / z): 673[M+H] +
[0080] 1 H NMR (DMAO-d 6 , 400MHz) δ: 0.88 (t, J=9.0Hz, 3H, CH 3 ), (t, J=8.4Hz, 3H, CH 3 ), 1.27-1.37 (m, 6H, CH 2 CH 2 CH 2 ), 1.57-1.61 (m, 2H, CH 2 ), 2.55-2.60 (m, 0.7H, CH 2 ), 2.68(t, J=14.4Hz, 2H, CH 2 ), 2.85(s, 0.3H, OH), 3.77(s, 3H, CH 3 ), 3.95-4.01 (m, 4H, 2CH 2 ), 4.22(t, J=14.4Hz, 2H, CH 2 ), 4.35-4.39 (m, 0.3H, CH), 4.61 (d, J=5.6Hz, 2H, CH 2 ), 6.76(d, J=8.8Hz, 2H, ArH), 6.89(d, J=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com