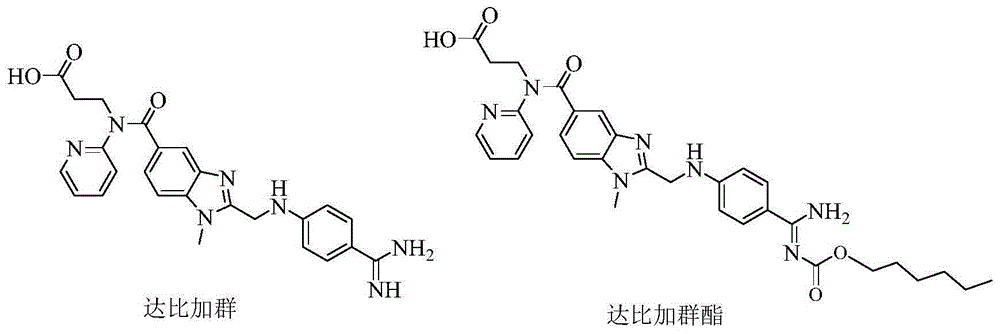
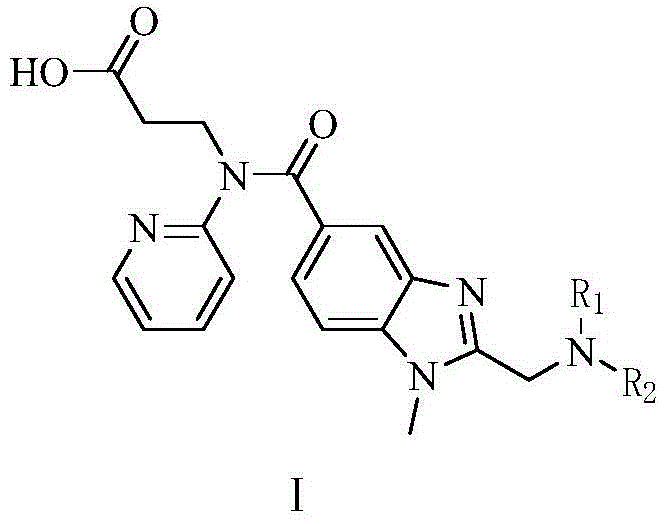
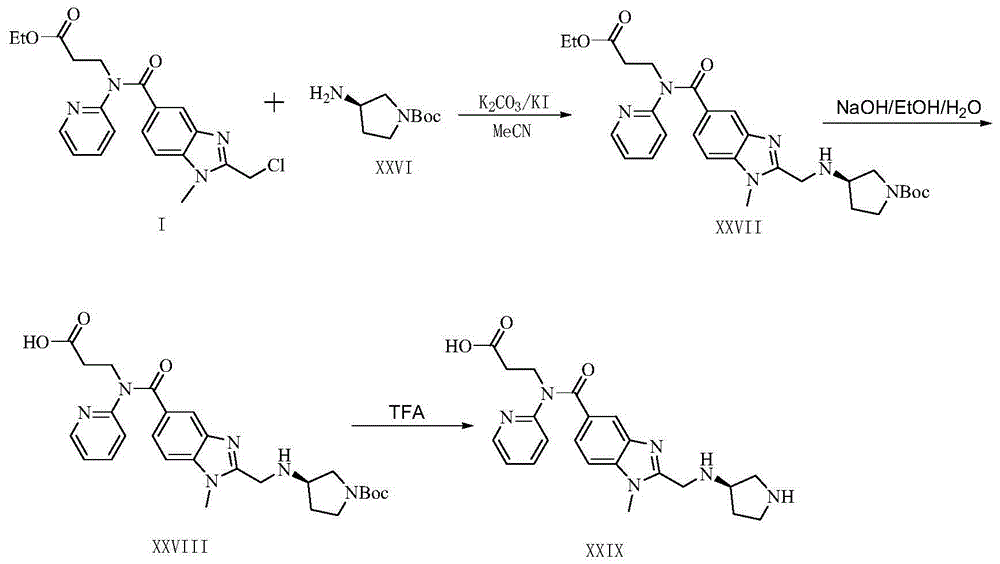
Dabigatran derivatives as well as preparation method and application thereof
A technology of dabigatran and its derivatives, which is applied in the field of medicine and can solve the problems of low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 13
[0048] Preparation Example 13-(2-((4-methoxyphenylamino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzimidazole-5-carbonylamino)propane Acid (III)
[0049]
[0050] Step (1): To 3-(2-(chloromethyl)-1-methyl-N-(pyridin-2-yl)-1H-benzimidazole-5-carbonylamino)propionic acid ethyl ester (I) (8.02g, 20mmol) in a stirred solution of acetonitrile (50ml), sequentially added 4-methoxyaniline (2.96g, 24mmol), potassium carbonate (5.53g, 40mmol), potassium iodide (0.34g, 2mmol). The mixture was stirred at 80°C for 5 hours. The solution was cooled to room temperature, filtered, and the solvent was removed under reduced pressure. The residue was partitioned between dichloromethane (200 mL) and water (150 mL), and the organic layer was separated. The aqueous phase was further extracted with dichloromethane (150 mL) and the combined organic extracts were combined. Dry over anhydrous sodium sulfate, filter and concentrate to give crude 3-(2-((4-methoxyphenylamino)methyl)-1-methyl-N-(pyridin...
preparation Embodiment 2
[0053] Preparation Example 2: 3-(2-((2-chlorothiophene-5-carbonylamino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzimidazole-5-carbonyl Preparation of amino)propionic acid
[0054]
[0055] Step (1) At 0-5°C, slowly add dropwise to a stirred solution of 2-aminoacetic acid (IV) (5g, 66mmol) in aqueous sodium hydroxide solution (3.8mol / L) and tetrahydroimidazole (40ml) Boc anhydride (14.5g, 66mmol), after the dropwise addition, rise to room temperature (about 25°C) for 24 hours, then lower to 0-5°C, adjust the pH to about 3 with 2N hydrochloric acid, dichloromethane (80mL*3) The aqueous phase was extracted, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain 10 g of crude product 2-(tert-butoxycarbonylamino)acetic acid (V).
[0056] Step (2) At room temperature (around 25°C), slowly add carbonyldiimidazole ( 5.4g, 33.3mmol), stirred for 0.5 hours, added 3-(3-amino-4-(methylamino)-N-(pyridin-2-yl) benzamido) propionic a...
preparation Embodiment 3
[0061] Preparation Example 3: 3-(2-((1H-indol-6-ylamino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzimidazole-5-carbonyl The preparation of amino)propionic acid (XV)
[0062]
[0063] Step (1): To 3-(2-(chloromethyl)-1-methyl-N-(pyridin-2-yl)-1H-benzimidazole-5-carbonylamino)propionic acid ethyl ester (I) (8.02g, 20mmol) in a stirred solution of acetonitrile (50ml), were added successively 6-aminoindole (XII) (3.17g, 24mmol), potassium carbonate (5.53g, 40mmol), potassium iodide (0.34g, 2mmol). The mixture was stirred at 80°C for 2 hours. The solution was cooled to room temperature, filtered, and the solvent was removed under reduced pressure. The residue was partitioned between dichloromethane (200 mL) and water (150 mL), and the organic layer was separated. The aqueous phase was further extracted with dichloromethane (200 mL) and the combined organic extracts were combined. Dry over anhydrous sodium sulfate, filter and concentrate to give crude 3-(2-((1H-indol-6-ylamino)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com