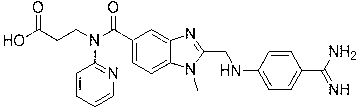
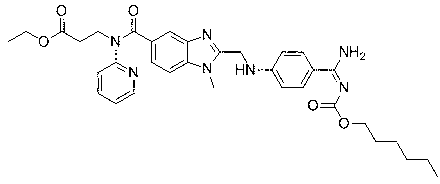
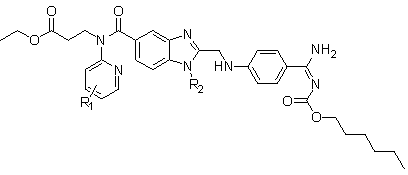
Dabigatran etexilate analogue with fluorine-containing group modified pyridine ring as center and synthesis method of analogue
A dabigatran etexilate and synthetic method technology, applied in the direction of drug combination, blood disease, extracellular fluid disease, etc., can solve the problems of increasing the risk of the preparation process, low oral bioavailability, high incidence of bleeding, etc., to achieve Reduce the difficulty and risk, shorten the synthesis time, and reduce the drug metabolism rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A dabigatran etexilate analog centered on a pyridine ring modified by a fluorine-containing group, with image 3 R in 1 for -F, R 2 for-C 2 h 5 For example, 3-[[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-ethyl-1H- Benzimidazol-5-yl]carbonyl](5-fluoropyridin-2-yl)amino]propanoic acid ethyl ester.
[0057] The above-mentioned a kind of dabigatran etexilate analog centered on a pyridine ring modified by a fluorine-containing group is 3-[[[2-[[[4-[[[(hexyloxy)carbonyl]amino]imino Methyl] phenyl] amino] methyl] -1-ethyl-1H-benzimidazol-5-yl] carbonyl] (5-fluoropyridin-2-yl) amino] the synthetic method of ethyl propionate, namely Using 5-fluoropyridin-2-ylamino as raw material, the final product is a fluorine-modified dabigatran etexilate analog through 9-step reaction synthesis, namely 3-[[[2-[[[4-[[[ (Hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-ethyl-1H-benzimidazol-5-yl]carbonyl](5-fluoropyridin-2-yl) Amino] ethyl propionat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com