Dabigatran etexilate novel intermediate, and preparation method and application thereof
A technology of dabigatran etexilate and catalytic reaction, applied in the direction of organic chemistry, etc., can solve the problems of unsuitability for industrial production, high price, and odor, and achieve high yield and low cost preparation, short reaction time, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
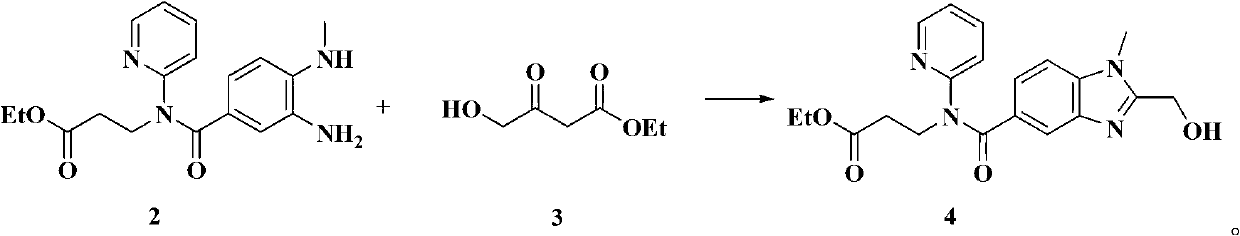
[0034] Embodiment 1: the preparation of formula 4 compound (method 1)
[0035] Heat 4.3g (0.0292mol) of compound of formula 3, 5.0g (0.0146mol) of compound of formula 2, 0.5g of ammonium chloride, and 30ml of ethanol to 65-70°C under nitrogen protection and stir for two hours. Chloromethane 100ml, water 100ml×2 washed, dried over anhydrous sodium sulfate, filtered and evaporated to dryness, added 30ml ethyl acetate, stirred to precipitate solid, filtered, and dried to obtain 4.8g of product, yield 86.0%.
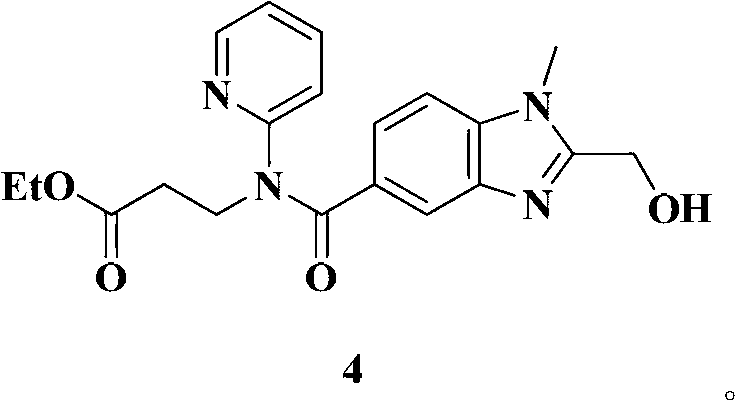
[0036] Melting point: 140-143℃ESI-MS(m):383[M+H] +
Embodiment 2
[0037] Embodiment 2: the preparation of formula 4 compound (method 2)
[0038] The compound of formula 2 (5.0g, 0.146mol), the compound of formula 5 (3.8g, 0.161mol), 0.5g of ammonium chloride, and 30ml of absolute ethanol were stirred and reacted at 65-70°C under nitrogen protection for 3h. Add 1.0 g of palladium-carbon catalyst with a content of 5%, and stir the reaction under normal pressure with hydrogen reflux, monitor by TLC until the reaction of the raw materials is complete, filter to remove the catalyst and inorganic salt, concentrate to dryness, add 20 ml of ethyl acetate and stir, filter to precipitate a solid, After drying, 5.0 g of the target product was obtained, with a yield of 89.6%.
Embodiment 3
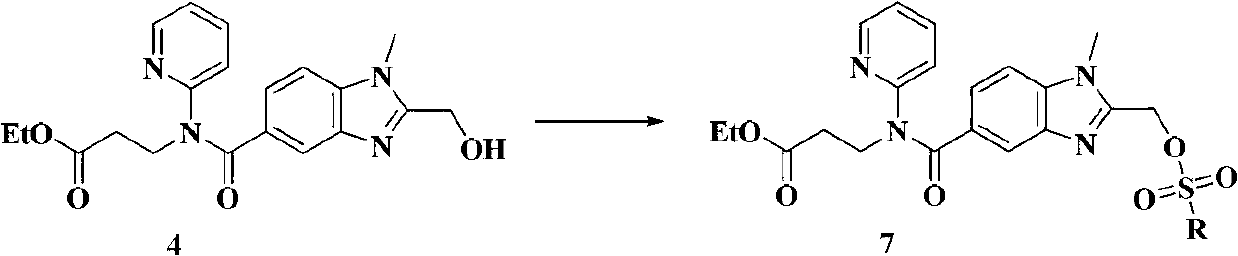
[0039] Embodiment 3: the preparation of formula 7 compound, R is methyl
[0040] Add the compound of formula 4 (5.0g, 0.0131mol) and triethylamine (3.3g, 0.0327mol) into 100ml of dichloromethane, add methanesulfonyl chloride (3.0g, 0.0262mol) dropwise within 2min at 5-10°C, and stir After reacting for 2 minutes, add 100ml of ice water and 2ml of ammonia water, stir for 5min, and separate the layers. The organic layer is washed with 100ml of saturated sodium bicarbonate and 100ml of saturated sodium chloride.
[0041] ESI-MS(m / z):461[M+H] +
[0042] Embodiment: 4: the preparation of dabigatran etexilate
[0043] Add formula 8 hydrochloride (4.0g, 0.0133mol) into a 100ml single-necked flask, add 20ml of 10% sodium hydroxide solution, 90ml of butyl acetate, add 50°C and stir for 30min, separate the layers, and extract the water layer once with 10ml of butyl acetate , the combined organic layers were washed once with 50 ml of saturated sodium bicarbonate and once with 100 ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com