Improved method for preparing Dabigatran etexilate
A technology of dabigatran etexilate and molar ratio, applied in the field of preparation of dabigatran etexilate, can solve problems such as waste acid treatment and industrial production troubles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
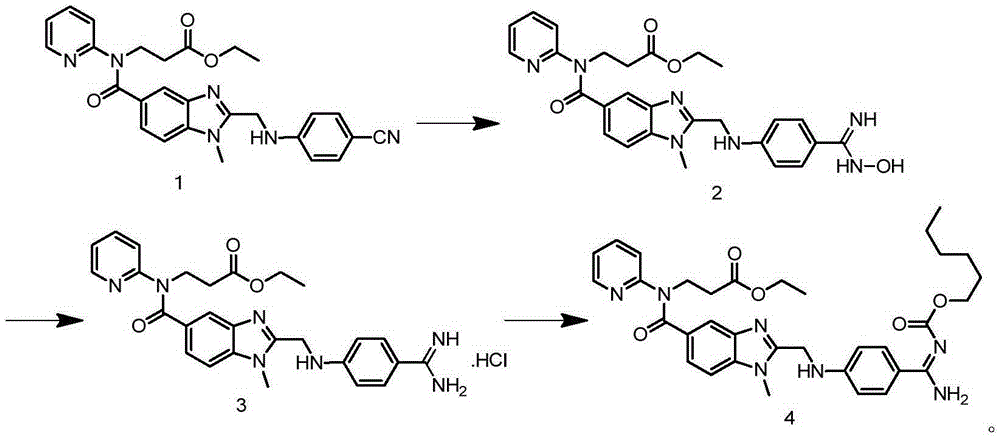
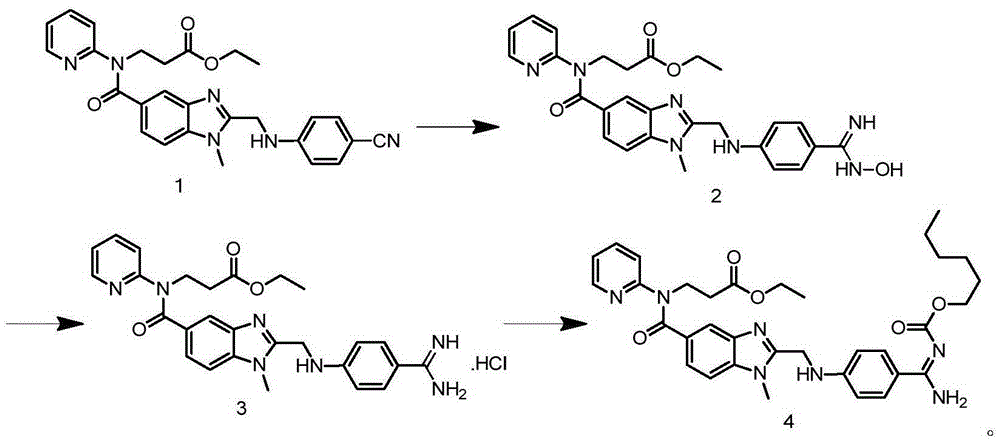
[0030] Add 250mL of absolute ethanol to the reaction bottle, add 72.4g of the compound of formula 1 under stirring, add 15.6g of hydroxylamine hydrochloride after dissolving, then raise the temperature to 58°C, slowly add 72.9g of 21% sodium ethylate in ethanol solution dropwise, and dropwise Afterwards, keep the reaction at 58°C for 23 hours, lower the temperature to 0-5°C and stir for 1 hour, filter, and wash the filter cake with 30 mL of absolute ethanol to obtain the compound of formula 2.
[0031] Add the above solid (that is, the compound of formula 2) into the reaction flask, add 300mL of purified water, stir and add 1.0g of 10% Pd / C, heat up to 70°C, slowly add 9.0g of glacial acetic acid dropwise, and stir for 3 hours after the drop is complete , filtered, added 16.8mL of concentrated hydrochloric acid to the mother liquor, concentrated until solids precipitated, added 180mL of acetone, stirred and cooled to below 5°C for crystallization for 1 hour, filtered, and vacuu...
Embodiment 2
[0034] Add 250mL of absolute ethanol to the reaction bottle, add 72.4g of the compound of formula 1 under stirring, add 15.6g of hydroxylamine hydrochloride after dissolving, then raise the temperature to 58°C, slowly add 72.9g of 21% sodium ethylate in ethanol solution dropwise, and dropwise Afterwards, keep the reaction at 58°C for 23 hours, lower the temperature to 0-5°C and stir for 1 hour, filter, and wash the filter cake with 30 mL of absolute ethanol to obtain the compound of formula 2.
[0035] Put the above solid into the reaction bottle, add 300mL of purified water, stir and add 21.0g of reduced iron powder, raise the temperature to 70°C, slowly add 9.0g of glacial acetic acid dropwise, stir and react for 3 hours after dropping, filter, add 16.8mL of mother liquor Concentrated hydrochloric acid, concentrated until solids precipitated, added 180mL of acetone, stirred and cooled to below 5°C for crystallization for 1 hour, filtered, and vacuum dried at 50°C to obtain 60...
Embodiment 3
[0038] Add 250mL of methanol to the reaction flask, add 72.4g of the compound of formula 1 under stirring, add 10.4g of hydroxylamine hydrochloride after dissolving, then raise the temperature to 55°C, slowly add 38.6g of 21% methanol solution of sodium methoxide dropwise, and keep React at 55°C for 25 hours, lower the temperature to 0-5°C and stir for 1 hour, filter, wash the filter cake with 30 mL of methanol, and obtain the compound of formula 2.
[0039] Add the above solid (that is, the compound of formula 2) into the reaction flask, add 300mL of purified water, stir and add 8.4g of reduced iron powder, raise the temperature to 50°C, slowly add 9.0g of glacial acetic acid dropwise, and stir for 5 hours after the dropwise reaction. Filtrate, add 16.8mL of concentrated hydrochloric acid to the mother liquor, concentrate until solid precipitates, add 160mL of acetone, stir and cool down to below 5°C for crystallization for 1 hour, filter, and vacuum dry at 50°C to obtain 61.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com