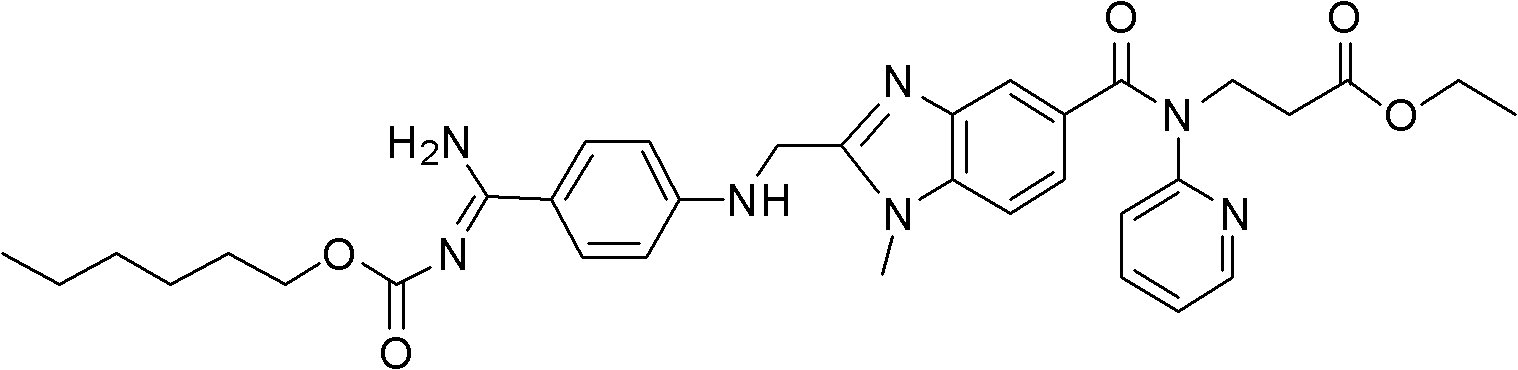
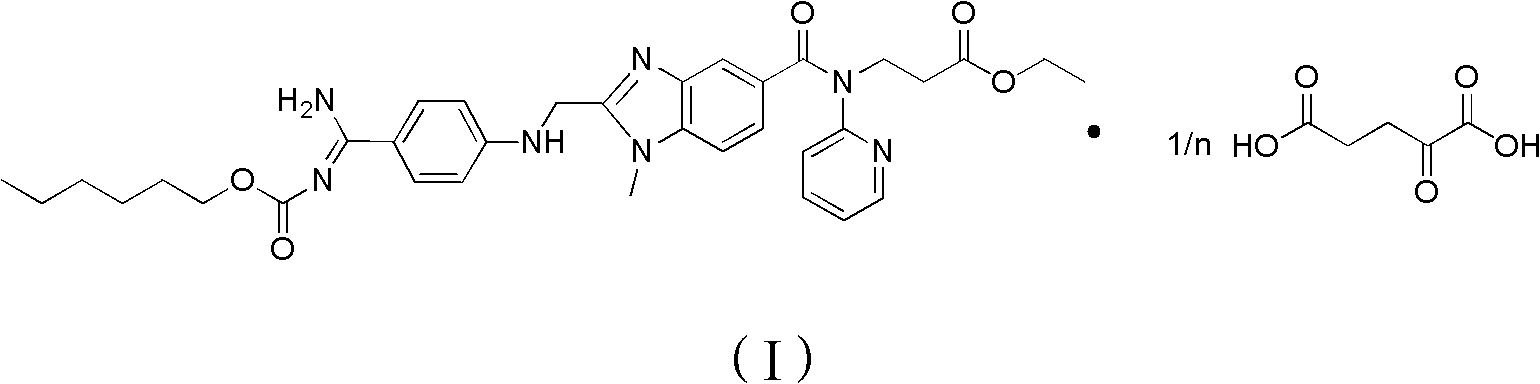
Dabigatran etexilate 2-ketoglutarate as well as preparation method and application thereof
A technology of dabigatran etexilate and ketoglutarate, which is applied in the field of acid addition salts of dabigatran etexilate, can solve the problems of poor stability and low bioavailability, and achieves improved water solubility and rapid dissolution process , the effect of high stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This example is used to illustrate the preparation of dabigatran etexilate 2-oxoglutarate of the present invention.
[0049] Add 1.6mmol of dabigatran etexilate and 1.6mmol of 2-oxoglutaric acid to 20ml of absolute ethanol at 20°C, mix and stir for 6 hours to form a salt, then crystallize at 20°C, filter, and use After washing with ethyl acetate and drying, ether was added for recrystallization to obtain 0.310 g of ether solvate of dabigatran etexilate 2-ketoglutarate. Measured ESI-MS (electrospray ionization-mass spectrometry) (m / z): 811[M+H] + .
[0050] The above-mentioned ether solvate is dried to obtain 0.296 g of dabigatran etexilate 2-ketoglutarate as a white solid. After calculation, in the above-mentioned ether solvate, the amount of dabigatran etexilate 2-ketoglutarate The content is 95.4wt%. The ether solvate contained 0.5 molecule of ether per molecule.
[0051] The above-mentioned dabigatran etexilate 2-oxoglutarate is measured:
[0052] ESI-MS(m / z): 7...
Embodiment 2
[0062] This example is used to illustrate the preparation of dabigatran etexilate 2-oxoglutarate of the present invention.
[0063] Add 3.2mmol of dabigatran etexilate and 1.6mmol of 2-oxoglutaric acid to 20ml of water at 30°C, mix and stir for 6 hours to form a salt, then crystallize at 30°C, filter, and wash with ethyl acetate , after drying, adding water for recrystallization, to obtain 0.450 g of dabigatran etexilate 2-oxoglutarate hydrate. Measured ESI-MS(m / z): 719[M+H] + .
[0064] The above-mentioned hydrate was dried to obtain 0.439 g of dabigatran etexilate 2-ketoglutarate as a white solid. After calculation, in the above-mentioned hydrate, the content of dabigatran etexilate 2-ketoglutarate was 97.5 wt%. Each molecule of the hydrate contains 1 molecule of water, that is, monohydrate.
[0065] The above-mentioned dabigatran etexilate 2-oxoglutarate is measured:
[0066] ESI-MS(m / z): 701[M+H] +
[0067] 1 H NMR (DMAO-d 6 , 400MHz) δ: 0.84 (t, J=9.0Hz, 3H, CH ...
Embodiment 3
[0076] This example is used to illustrate the preparation of dabigatran etexilate 2-oxoglutarate of the present invention.
[0077] Add 4.8mmol of dabigatran etexilate and 1.6mmol of 2-oxoglutaric acid to 20ml of absolute ethanol at 0°C, mix and stir for 6 hours to form a salt, then crystallize at 0°C, filter, and use acetic acid After washing with ethyl ester and drying, ethylene glycol dimethyl ether was added for recrystallization to obtain 0.420 g of dabigatran etexilate 2-ketoglutarate as a white solid.
[0078] The above-mentioned dabigatran etexilate 2-oxoglutarate is measured:
[0079] ESI-MS(m / z): 677[M+H] +
[0080] 1 H NMR (DMAO-d 6 , 400MHz) δ: 0.84 (t, J=9.0Hz, 3H, CH 3 ), 1.09(t, J=8.4Hz, 3H, CH 3 ), 1.28-1.32 (m, 6H, CH 2 CH 2 CH 2 ), 1.55-1.60 (m, 2H, CH 2 ), 2.41-2.48 (m, 0.7H, CH 2 ), 2.66(t, J=14.4Hz, 0.7H, CH 2 ), 2.88-2.92 (m, 2H, CH 2 ), 3.76 (s, 3H, CH 3 ), 3.94-4.04 (m, 4H, 2CH 2 ), 4.20(t, J=14.4Hz, 2H, CH 2 ), 4.60 (d, J=5.6Hz, 2H, CH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com