Synthesis method of dabigatran etexilate
A dabigatran etexilate and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of cumbersome process and purification steps, difficult industrialization promotion, high cost, etc., and achieve avoidance of column chromatography separation, easy availability of raw materials, and route simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
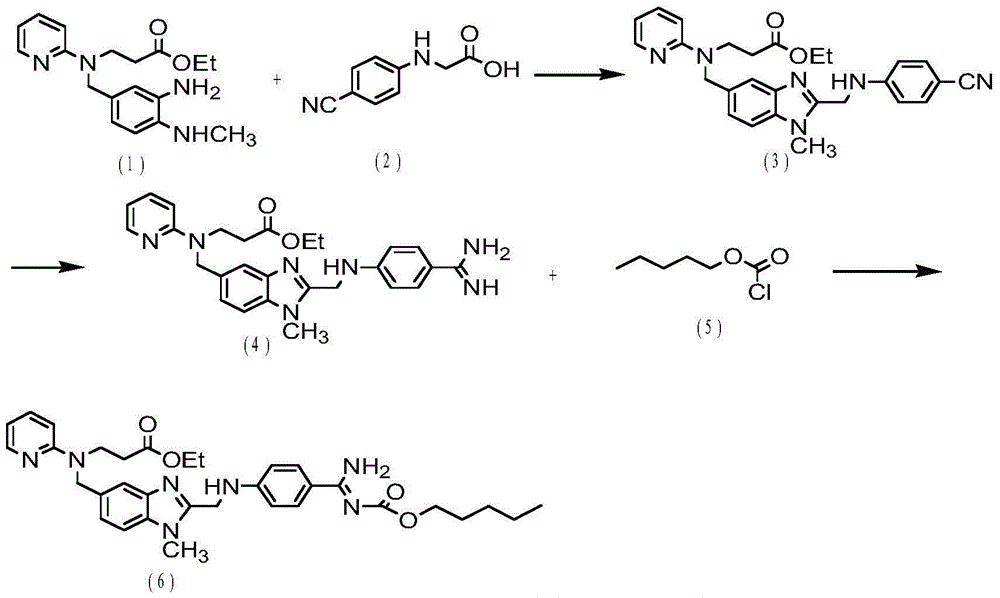
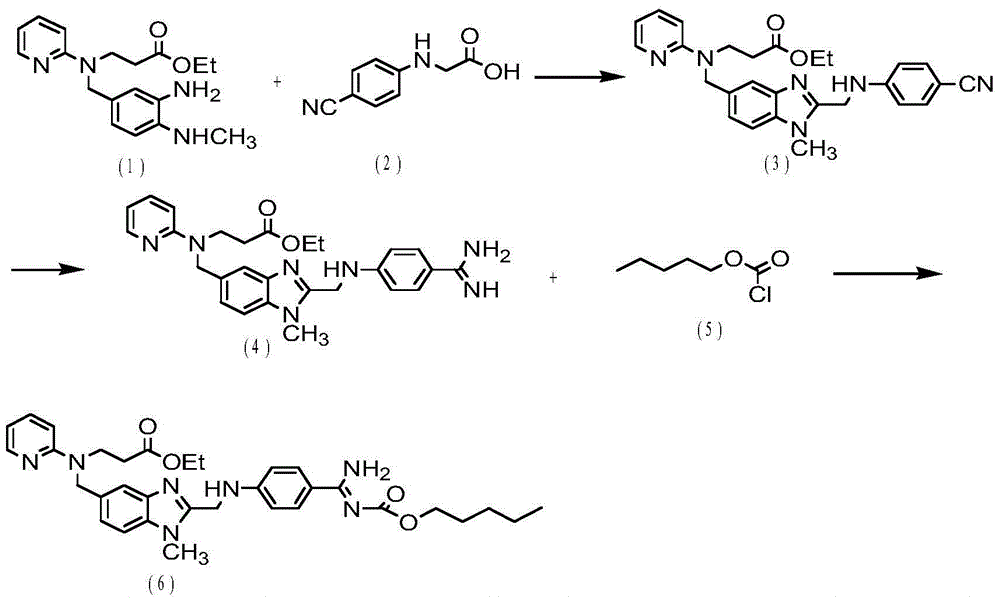
[0021] Example 1. Intermediate 3-({2-[(4-cyano-phenylimine)-methylene]-1-methylene-1H-benzimidazole-5-carbonyl}-pyridine-2- Synthesis of ethyl imine) propionate (3)
[0022] Add 3.35g (18.98mmol) N-(4-cyanophenyl)glycine (2) into 40ml of dichloromethane, stir for 10 minutes and cool down to 0-5°C, add 2.57g (18.98mmol) N,N -Carbonyldiimidazole (abbreviated as HoBt), after stirring for 20 minutes, add 3.64g (18.98mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt (abbreviated as EDCl), and continue to stir for 1 hour , slowly warmed to room temperature.
[0023] Then 5g (14.6mmol) 3-[(3-amino-4-methylaminobenzoyl) (pyridin-2-yl) amino] ethyl propionate (1) was dissolved in 30ml of dichloromethane, 15- Add dropwise to the above solution for 20 minutes, stir overnight at room temperature, then evaporate the solvent to dryness, add ethyl acetate (analytical grade) and water for extraction, take the organic layer and dry it with anhydrous sodium sulfate, filter, and evapora...
Embodiment 2
[0030] Example 2. Intermediate 3-({2-[(4-cyano-phenylimine)-methylene]-1-methylene-1H-benzimidazole-5-carbonyl}-pyridine-2- Synthesis of ethyl imine) propionate (3)
[0031] Add 3.09g (17.52mmol) N-(4-cyanophenyl)glycine (2) into 40ml of dichloromethane, stir for 10 minutes and cool down to 0°C, add 2.37g (17.52mmol) N,N-carbonyl Diimidazole (abbreviated as HoBt), after stirring for 22 minutes, add 3.36g (17.52mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt (abbreviated as EDCl), continue to stir for 1 hour, slowly Bring to room temperature.
[0032] Then 5g (14.6mmol) 3-[(3-amino-4-methylaminobenzoyl) (pyridin-2-yl) amino] ethyl propionate (1) was dissolved in 30ml of dichloromethane, 15- Add dropwise to the above solution for 20 minutes, stir overnight at room temperature, then evaporate the solvent to dryness, add ethyl acetate (analytical grade) and water for extraction, take the organic layer and dry it with anhydrous sodium sulfate, filter, and evaporate the s...
Embodiment 3
[0039] Example 3. Intermediate 3-({2-[(4-cyano-phenylimine)-methylene]-1-methylene-1H-benzimidazole-5-carbonyl}-pyridine-2- Synthesis of ethyl imine) propionate (3)
[0040] Add 3.09g (17.52mmol) N-(4-cyanophenyl)glycine (2) into 40ml of dichloromethane, stir for 10 minutes and cool down to -5-0°C, add 2.37g (17.52mmol) N, N-carbonyldiimidazole (abbreviated as HoBt), after stirring for 20 minutes, add 3.36g (17.52mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt (abbreviated as EDCl), continue to stir for 1 hour, slowly warming to room temperature.
[0041] Then 5g (14.6mmol) 3-[(3-amino-4-methylaminobenzoyl) (pyridin-2-yl) amino] ethyl propionate (1) was dissolved in 30ml of dichloromethane, 15- Add dropwise to the above solution for 20 minutes, stir overnight at room temperature, then evaporate the solvent to dryness, add ethyl acetate (analytical grade) and water for extraction, take the organic layer and dry it with anhydrous sodium sulfate, filter, and evaporate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com