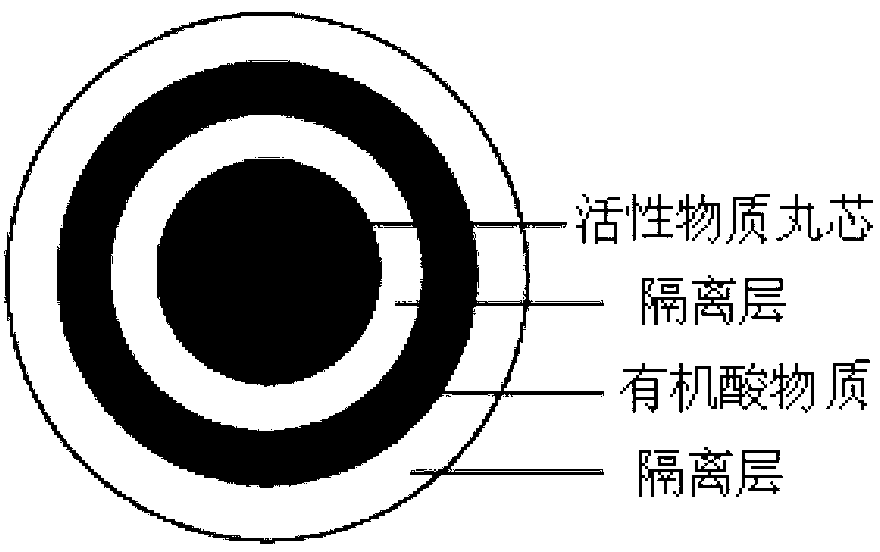
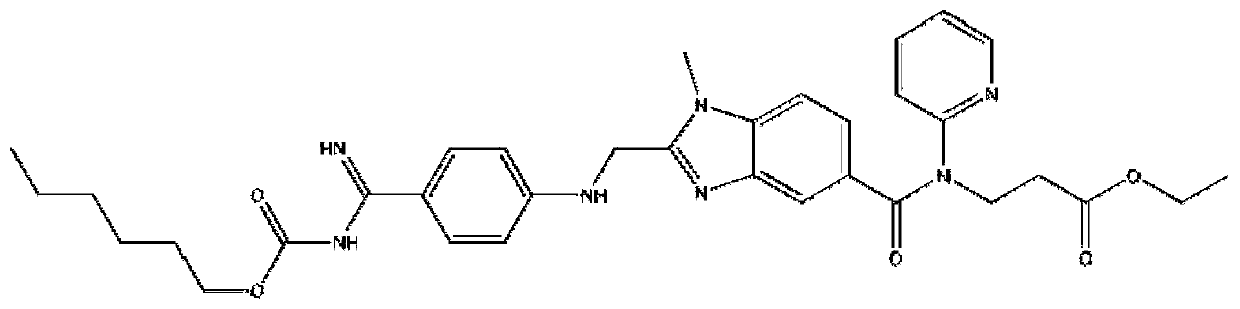
Pharmaceutical composition containing dabigatran etexilate or salt and hydrate thereof
A hydrate and composition technology, applied in the field of medicine, can solve the problems of low yield of finished products, small dosage of medicine, difficult to control dosage of medicine, etc., and achieve good storage stability, good dissolution and dissolution, and bioavailability effect. outstanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 preparation of pellet core material containing active substance
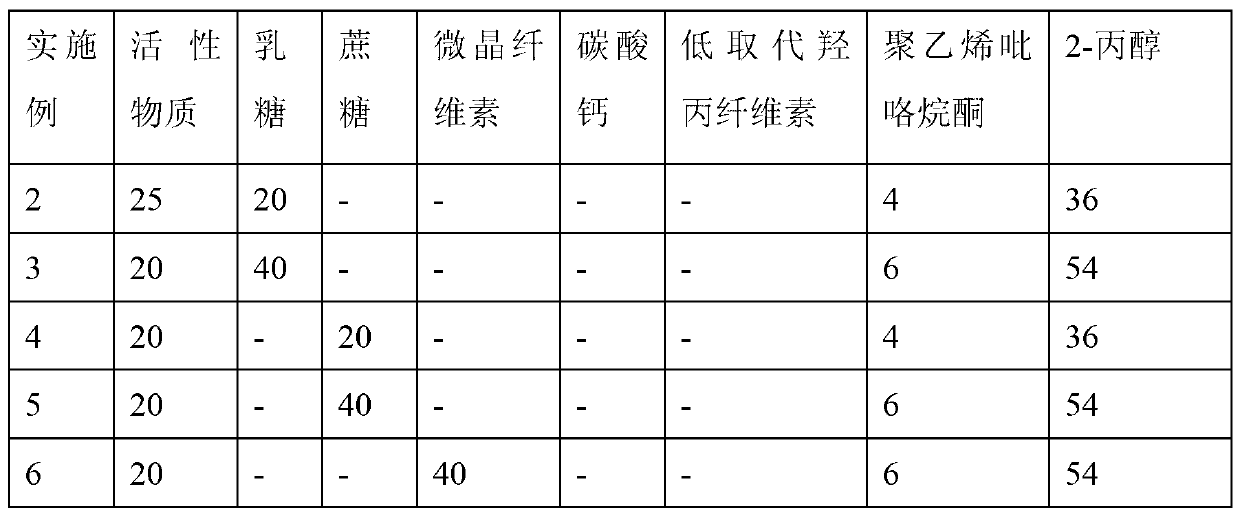
[0034] Example
active substance
2-propanol
1
20
20
4
36
[0035] Weighing 4g wrong! Reference source not found. Dissolved in 36 g of 2-propanol. Mix 20g of dabigatran etexilate mesylate (active substance) and 20g of lactose with mechanical stirring, then add 4g of wrong! Reference source not found. A solution of 2-propanol (36g) was mixed into a suitable soft material and passed through an extrusion spheronizer to form roughly spherical particles. Then the spherical active material core material is dried on a fluidized bed at a material temperature of 30-35° C. to obtain active material ball cores.
[0036] The core material was separated using a drum screener with perforated plates of nominal mesh size 0.6-1.0 mm. The product fraction between 0.6-1.0 mm was used in the remainder of the process.
Embodiment 2-10
[0037] Embodiment 2-10 preparation of active substance ball core material
[0038]
[0039]
[0040] Examples 2-10 Active substance pellet core materials were prepared in the same manner as in Example 1.
Embodiment 11-17
[0041] The preparation of embodiment 11-17 active substance ball core material
[0042]
[0043] Examples 11-17 Active substance pellet core materials were prepared in the same manner as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com