Dabigatran etexilate benzene sulfonate as well as preparation method and application thereof
A technology of dabigatran etexilate and besylate, applied in the field of medicine, can solve the problems of poor absorption, large distribution coefficient, difficult transport and the like, and achieve the effects of improving drug efficacy and broad prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
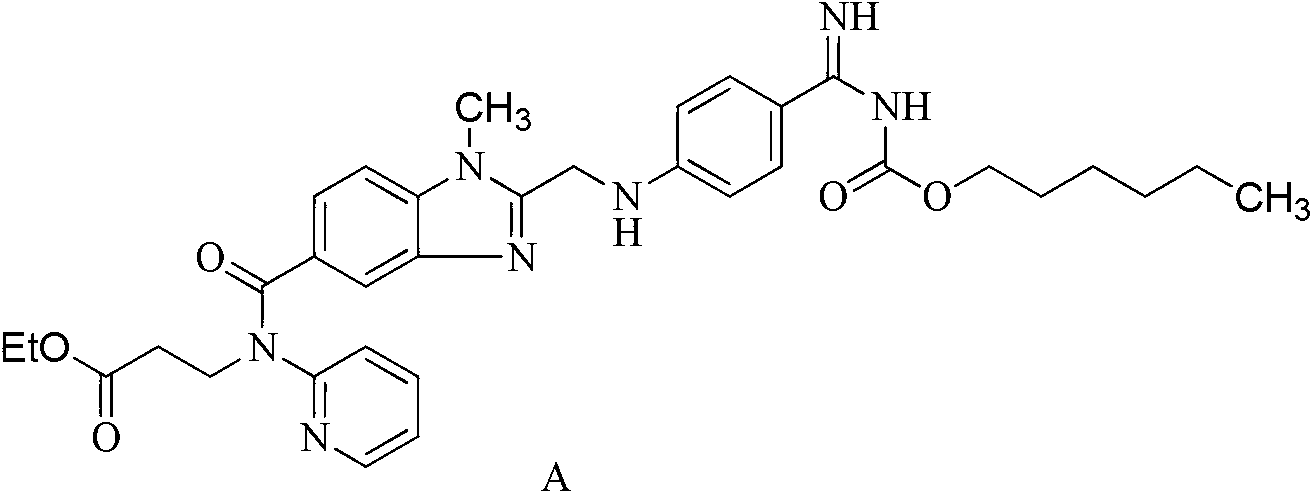
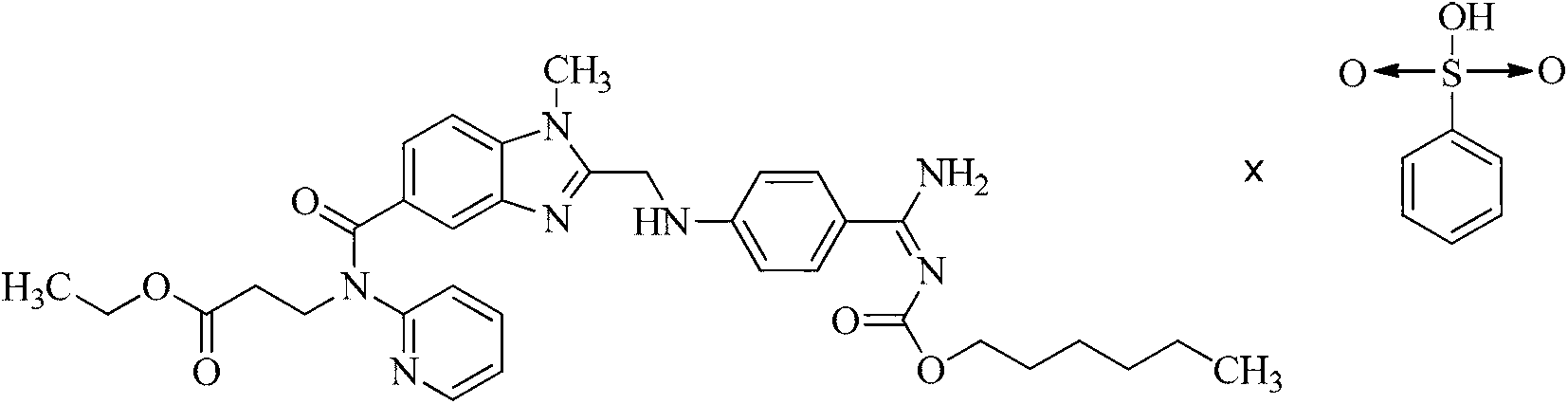
[0024] Example 13-[(2-{4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1Hbenzimidazole-5-carbonyl)-pyridine- Preparation of 2-yl-amino]-propionate ethyl benzenesulfonate
[0025] Include steps:
[0026] 3-[(2-{4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1Hbenzimidazole-5-carbonyl)-pyridine-2 -Base-amino]-propionic acid ethyl ester 5g (0.008mol) was dissolved in 350ml of acetone, at room temperature, stirred and added 1.56g (0.0096mol) benzenesulfonic acid solid, after stirring at room temperature for 5h, the ice bath cooled to 0 Stir at -4°C for 24h. The precipitated solid was filtered, washed with an appropriate amount of acetone, and the product was vacuum-dried at a maximum temperature of 50° C. for 4 hours to obtain 5.10 g (81%) of a light yellow powder. HPLC determined that the light yellow powder was the target product with a purity of 99.6%.
Embodiment 23
[0027] Example 23-[(2-{4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1Hbenzimidazole-5-carbonyl)-pyridine- Preparation of 2-yl-amino]-propionate ethyl benzenesulfonate
[0028] Include steps:
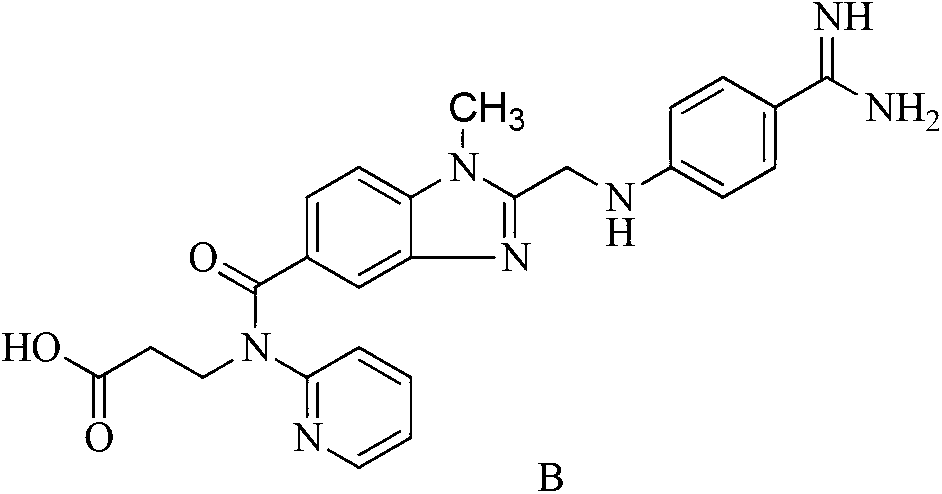
[0029] 3-[(2-{4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1Hbenzimidazole-5-carbonyl)-pyridine-2 5 g (0.008 mol) of ethyl propionate was dissolved in 500 ml of tetrahydrofuran, and 1.3 g (0.008 mol) of benzenesulfonic acid solid was added with stirring at 45 ° C. After stirring at room temperature for 4 h, the temperature was cooled to Stir at 0-4°C for 28h. 500ml of ether was added to the reaction solution to precipitate a solid. The precipitated solid was filtered, washed with an appropriate amount of acetone, and the product was vacuum-dried at a maximum temperature of 50° C. for at least 4 hours to obtain 5.04 g (80%) of a light yellow powder. HPLC determined that the light yellow powder was the target product with a purity of 99.5%...
Embodiment 33
[0030] Example 33-[(2-{4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1Hbenzimidazole-5-carbonyl)-pyridine- Preparation of 2-yl-amino]-propionate ethyl benzenesulfonate
[0031] Include steps:
[0032] 3-[(2-{4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1Hbenzimidazole-5-carbonyl)-pyridine-2 -Base-amino]-propionic acid ethyl ester 5g (0.008mol) was dissolved in 350ml of methanol, and at 50-60°C, 2.6g (0.016mol) of benzenesulfonic acid solid was added with stirring, and after stirring at room temperature for 3h, the ice bath Cool down to 0-4°C and stir for 32h. The precipitated solid was filtered, washed with an appropriate amount of acetone, and the product was vacuum-dried at a maximum temperature of 50° C. for at least 4 hours to obtain 4.9 g (78%) of a light yellow powder. HPLC confirmed that the light yellow powder was the target product with a purity of 99.3%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com