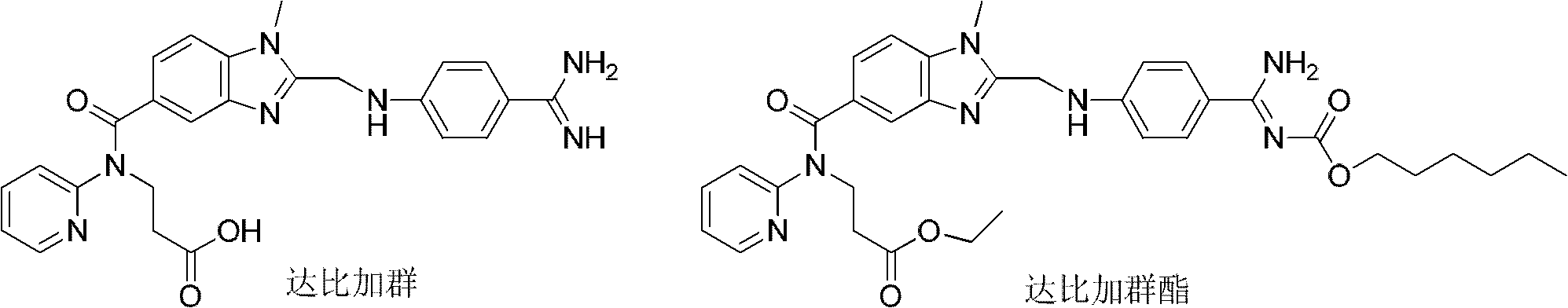
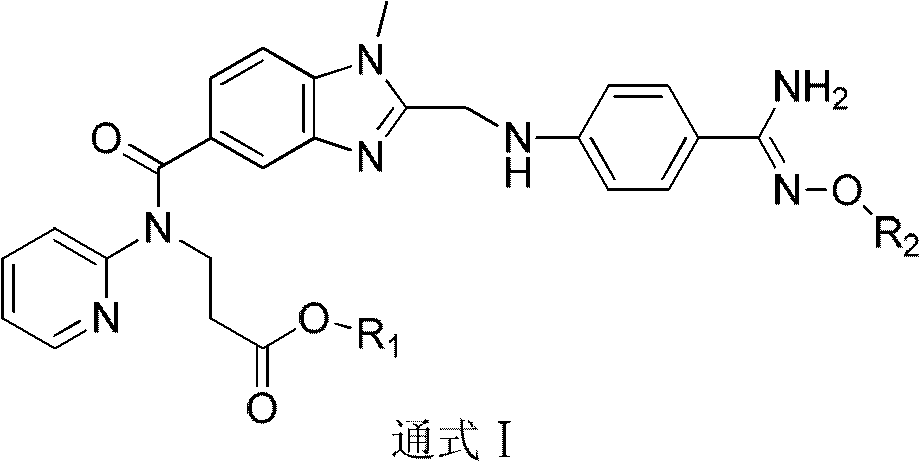
Dabigatran derivatives and preparation method thereof
A technology of ester derivatives and dabigatran, applied in the field of medicine, can solve the problems of low oral bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: 3-[[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H-benzene Preparation of ethyl imidazol-5-yl]carbonyl](pyridin-2-yl)amino]propionate (dabigatran etexilate)
[0066] 1) Synthesis of 3-(pyridine-2-imine)-ethyl propionate (2)
[0067] Under the protection of nitrogen, ethyl acrylate (13.8g, 0.14mol) was added to compound 2-aminopyridine (11.2g, 0.12mol), stirred and refluxed at 10°C for 24h, the precipitate was filtered off, the residue was concentrated and purified by column chromatography 10 g of a white solid were obtained.
[0068] 2) Synthesis of 4-aminomethyl-3-nitro-benzoic acid (4)
[0069] 50 mL of 33% methylamine aqueous solution was added to 4-chloro-3-nitrobenzoic acid (10.0 g, 0.05 mol), and the system was reacted at 110° C. for 6 hours. The pH was adjusted to 4 by adding glacial acetic acid. After standing overnight, a large amount of yellow solid precipitated out, and the solution was filtered off to obtain 7...
Embodiment 2
[0082] Example 2: N-[[2-(((4-(((2-aminopropyl)oxy)amidino)-phenyl)-amino-methyl)-1-methyl-1H-benzo Preparation of imidazol-5-yl)-carbonyl]N-(pyridin-2-yl)-β-alanine methyl ester (I1)
[0083] 1) N-[[2-(((4-cyano-phenyl)-amino-methyl)-1-methyl-1H-benzimidazol-5-yl)-carbonyl]N-(pyridine-2 Synthesis of -yl)-β-alanine methyl ester (10a)
[0084] Dissolve 5.0 g of intermediate 8 in 200 mL of ethanol, add 10 mL of 1N sodium hydroxide solution, stir the reaction at room temperature until hydrolysis is complete, evaporate to dryness, dissolve in 20 mL of DMF, add 1.76 g of methyl iodide, stir at room temperature for 24 hours, concentrate, and column 4.0 g of the target intermediate were isolated.
[0085] 2) N-[[2-(((4-(N-hydroxyamidino)-phenyl)-amino-methyl)-1-methyl-1H-benzimidazol-5-yl)-carbonyl]N Synthesis of -(pyridin-2-yl)-β-alanine methyl ester (11a)
[0086] Dissolve 4.0g of intermediate 10a in 100mL of ethanol, cool to 0°C, pass in dry hydrogen chloride gas to saturation,...
Embodiment 3
[0097] Example 3: Evaluation of Anticoagulant Activity - Determination of Activated Partial Thromboplastin Time (aPPT)
[0098] Kunming mice with a mass of 18-20 g were divided into random groups, 10 in each group, and fasted overnight. Dabigatran etexilate and target compound to be tested are suspended or dissolved in the aqueous solution of 1% sodium carboxymethyl cellulose, made into the concentration of 1mg / mL, according to the dose of 10mg / Kg (converted into dabigatran calculation) ) for intragastric administration, half an hour later blood was collected by cardiac puncture, 4% sodium lyciate solution was added to a final concentration of 0.4% for anticoagulation, centrifuged at 12000r / min for 5 minutes, 0.1mL of plasma was taken, 0.1mL of aPPT reagent was added, and 37°C After pre-warming for 3 minutes, add 0.1 mL of calcium chloride solution pre-warmed at 37°C, and measure the coagulation time with a platelet aggregation coagulation factor analyzer, which is the aPPT va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com